The way we think about obesity has undergone a profound shift in recent years. Previously viewed as an individual lifestyle choice, it is now recognised as a complex disease influenced by genetics, biology, psychosocial factors and the environment. We also know that it affects a huge proportion of people.
The World Health Organization (WHO) estimates that nearly two billion adults are overweight or obese. And numbers now soaring dramatically in low-, middle- and high-income countries alike.
This is a major problem: excess body fat (adipose tissue) increases the risk of diseases such as diabetes, heart disease, dementia, cancer, non-alcoholic fatty liver disease and kidney failure.
Fortunately, recent discoveries are helping us understand more about obesity. For instance, over the past 30 years, we have learned that energy balance and eating behaviour are regulated not in the stomach, but in the brain.
Scientists have discovered hundreds of genes that affect our bodies’ weight regulation, some of which predispose us to obesity. We also know that maintaining weight loss is difficult for many people because of the body’s natural responses. Weight loss causes our metabolisms to slow down and hunger hormones to increase.
This evolving understanding has driven significant efforts to find effective treatments for people living with obesity, who are often stigmatised and discriminated against.
Traditionally, weight-loss strategies have been based on either surgery or lifestyle changes such as diet and exercise. Surgery can be an effective option, but it’s not without risks, and can potentially have long-term or irreversible effects. It’s also expensive, and availability is limited.
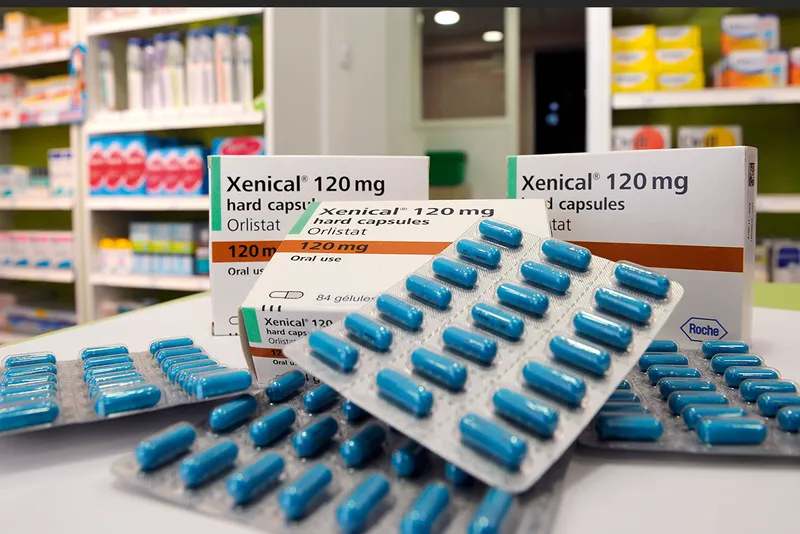
Could weight-loss drugs soon offer a silver bullet? So far, they haven’t proved to be very effective. Take orlistat, for instance, which functions by inhibiting the absorption of fat in the intestines. Though not unsafe, it produces a mere 3 per cent body weight loss and necessitates dietary restrictions.
Others, such as combined phentermine-topiramate (not licensed in the UK), act on the central nervous system to activate ‘fight or flight’ responses and increase energy expenditure. This, as you might guess, may pose risks to the cardiovascular system.
Should we impede appetite?
The future of weight-loss medicine may lie in new gut-hormone therapies called incretins. These peptides – chains of amino acids – are naturally produced in the body in response to food intake, and can help regulate appetite via the gut-brain axis. Examples include glucagon, gastric inhibitory peptide, and glucagon-like peptide 1 (GLP-1).
Current medicines licensed for obesity, such as semaglutide (sold under the brand names Wegovy in the UK and Ozempic in the US), activate the GLP-1 hormone pathway. Upcoming ‘twincretin’ medicines, such as Tirzepatide, go further by combining two hormones delivered by one pen injector.
These therapies produce significant body weight loss, improve conditions such as high blood sugar, and have been shown in large clinical trials to produce largely manageable side effects. Importantly, Wegovy has been shown to reduce the risk of cardiovascular events in a promising preview of what we hope is to come – effective obesity management and timely disease prevention.
However, these new medications are not without challenges.
What are the challenges?
First, most must be injected by patients, many of whom are averse to doing so. Second, clinical trials have so far shown that weight gain restarts when medication is stopped. And that’s before we look at the high costs of manufacture and distribution for drugs that need to be stored in cool conditions. Added to that is the fact that demand heavily outweighs supply.
This is a big problem: we know that people who live with obesity are more likely to be in lower socioeconomic groups, so expensive solutions will likely widen health inequalities. Ultimately, any ‘magic pill’ for weight loss must be both safe and effective. The side effects should be manageable and there should be great outcomes for patients in terms of overall physical and mental health.
It also needs to be cheap to produce, so that all patients who need it, can access it. It wouldn’t just affect numbers on weighing scales but would improve patients’ quality of life, too.
More innovative weight-loss pills may just be around the corner. With industry giants such as Novo Nordisk, Eli Lilly, Pfizer, Boehringer Ingelheim and AstraZeneca leading the charge, numerous new medications are anticipated in the next 30 years.
Orforglipron looks promising
Eli Lilly appears to have the most promising oral medication in its pipeline: a small-molecule nonpeptide named orforglipron. Currently undergoing phase 3 clinical trials, the medication signals fullness to the brain. It stimulates insulin release and slows the speed at which food travels through the digestive tract, making recipients feel fuller for longer.
Phase 2 clinical trials showed nearly 15 per cent body weight loss at 36 weeks. However, it’s hard to say exactly when these drugs will be released.
Predicting the timeline for such breakthroughs is challenging, not least because of the difficulties in bringing new medications to the market. It’s a time-consuming and expensive process with few successes.
In addition, weight-loss medication has historically produced severe side effects. So there is an emphasis on ensuring safety over longer-term use.
The future of weight-loss pills
Despite these challenges, the future of obesity treatment is exciting and intriguing because developing weight-loss medication involves navigating the complexities of human biology.
One promising avenue might be developing medication that stimulates natural fat-burning pathways, perhaps manipulating certain types of fat. For instance, brown adipose tissue can break down glucose and fat molecules to create heat and maintain body temperature.
Could a drug be made to harness this effect? Other hormones under investigation include amylin, secreted at the same time as insulin. Finally, advancing technologies such as gene therapy, nanotechnology and CRISPR gene-editing offer potential pathways for developing effective treatments.
Whatever advances are made, though, a ‘magic’ weight-loss pill won’t provide a single effective way to combat obesity within the next few decades. It’s important to note that appropriately supported, clinically-led lifestyle changes can be very effective for some patients, as can surgery. Patients living with obesity have the right to access the right treatment pathway for them. So we must work to remove the stigma attached to obesity.
A pill could be an effective tool, but just one of many. It can’t replace healthy habits and psychological wellbeing, nor can it address the socioeconomic factors that drive health inequalities.
What else can we do?
Psychological factors, such as disordered eating and trauma, are other crucial targets in the treatment of obesity. Yet they’re currently overlooked in favour of pharmacological solutions. So, although the promise of a weight-loss pill is certainly exciting, it should be viewed as one potential option in a larger toolbox rather than a standalone solution.
Ultimately, the development of a safe and effective weight-loss pill still faces multiple challenges. These range from biological and psychological complexities to practical considerations including cost.
Previous failures to recognise obesity as a chronic disease, plus widespread prejudice against those living with obesity, have hindered progress. With the advent of new medications and a focus on the gut-brain axis, however, there’s cause for real hope.
Read more:

