The idea of using patients’ own immune cells to fight cancer is over a hundred years old. In the late 19th century, an American doctor called William Coley attempted to treat cancer by stimulating the immune system with dangerous bacteria, arguably the first example of what is now known as immunotherapy. His procedure appeared to shrink some patients’ tumours, but was criticised as unsafe and largely forgotten about. Chemotherapy and radiotherapy became the focus of cancer research and the standard tumour treatment for the rest of the 20th century.
Now, however, immunotherapy is at the forefront of cancer research again.
Our immune system is constantly detecting and destroying infected or defective cells in order to keep us healthy. When defective cells somehow evade this system and start to multiply in the body, we call that cancer. Cancer cells make it difficult for the immune system to work in many ways – they hide their tell-tale proteins, disguise themselves as healthy cells, and release chemical signals to slow down the immune system. Once cancer cells have grown into a tumour, it then becomes physically difficult for immune cells to penetrate and act within this hostile microenvironment.
Read more 2019 science breakthroughs:
Drugs made from antibodies, which help the immune system target cancerous cells, have been in use since the late 1990s. More recently, substances have been developed which turn off the chemical signals that tumours release to dampen the immune system.
In 2017, a handful of exciting new treatments were approved for use which are made from patients’ own modified immune cells instead of toxic chemicals. Known as CAR T-cell therapies, these treatments involve extracting immune cells from the patient, genetically modifying them to express molecules found on the surface of the cancer, then injecting millions of them back into the patient’s blood stream. These special cells, known as T-cells, then help orchestrate an immune system attack on the cancer cells en masse.
The holy grail
But the elusive holy grail of cancer immunotherapy is the so-called ‘cancer vaccine’. The idea here is to ‘teach’ the immune system to attack cancer cells in the same way a vaccination teaches the immune system how to recognise and deal with a certain virus. Not only would this help the body destroy a tumour, but it should help the immune system detect and neutralise any similar cancer cells that return in future.
Yet despite decades of research, cancer vaccines are only just starting to show promise in clinical trials. The therapies involve presenting the immune system with a chemical fragment of the tumour, just like when a weakened version of a virus or bacteria is presented to the body in a conventional vaccination. Finding a suitable molecular fragment from the tumour that will provoke a powerful but safe immune response (known as an antigen) has proven difficult.

Firstly, it must be an antigen not found anywhere else in the body, or the immune system could start to attack healthy cells. This immediately rules out around 95 per cent of tumour antigens. Even once a suitable, tumour-specific antigen is found, there’s no guarantee the immune system will react to it. After all, for the tumour to have grown, the cancerous cells and all their proteins must already be successfully evading the immune response somehow. Cancerous cells and tumours often release substances that prevent the immune system from working properly.
A new approach is to combine cancer vaccines with other substances that block cancer’s ability to manipulate and evade the immune system. These results have been much more promising, and experts believe this combination therapy will lead to a stream of cancer vaccines approved for use in 2019 and beyond.
“Our ideas about the design of cancer vaccines have changed,” says Haval Shirwan, a cancer immunotherapy expert and professor of immunology at the University of Louisville. “Using the right antigen alone is not enough: you have to also have a substance that helps the immune system overcome the evasive mechanisms that are stopping it from fighting the cancer itself.
These substances are known as checkpoint inhibitors. They block the chemical signals that normally act as brakes on the immune system, but can be used by cancer to suppress the immune response around a tumour. Checkpoint inhibitors hold a lot of potential. Indeed, the 2018 Nobel Prize in Physiology or Medicine was awarded to Dr James Allison and Dr Tasuku Honjo for their work on them.
“After a period of great doubt [about cancer vaccines], there is a great deal of excitement again,” says Christian Ottensmeier, professor of experimental cancer medicine at the University of Southampton. He says cancer researchers now better understand how to ‘package’ cancer vaccines so that the immune system recognises the key antigen as a danger, provoking immune cells to mount an attack. “It’s all about the tricks you use to make your antigen interesting to the immune system,” he says, suggesting cancer vaccines packaged within viruses, or even based on whole tumour cells, may be more effective.
Despite the progress being made, there is still much more to learn about how different people’s genetic make-up affects how they will respond to different treatments. In the future, powerful algorithms could help doctors quickly determine which antigen from an individual tumour is the most likely to provoke an effective immune response, and which are the best combinations of additional immune system boosters for that particular patient.
Targeting autoimmune disorders
The revolution in our understanding of the immune system is also helping researchers develop treatments for other diseases, too. In autoimmune disorders, the immune system mistakenly attacks healthy cells, causing chronic ill health. Here, researchers aim to do almost the exact opposite of cancer immunotherapy: instead of boosting immune system cells aiming for a specific target, they want to dampen down or switch off immune cells that are mistakenly attacking a target in the body.
Common autoimmune diseases include type 1 diabetes (caused by the immune system destroying insulin-producing cells), rheumatoid arthritis (caused by an inflammatory immune response in the joints) and multiple sclerosis (caused by immune system-related damage to the protective coating of nerves).
Until now, these diseases have been treated by just trying to provide relief from the symptoms or by dampening the whole immune system, which makes patients vulnerable to other disease and infection. A new approach is to identify the specific immune cells that are mistakenly attacking antigens in the body, and teach them that the molecules they think are dangerous are not.
A genome could be sequenced and an analysis done on the likelihood of an individual developing a disease, before it happens
“The approach is similar to the immunotherapies that have been used to ‘desensitise’ people to allergens,” says Professor David C Wraith, director of the Institute of Immunology and Immunotherapy at Birmingham University. He believes these ‘antigen-specific’ therapies for autoimmune diseases could be rolled out in around five years. They could even be used to prevent these diseases ever developing in people who are genetically at risk.
“You can imagine a day when everybody’s genome is sequenced and you can do a risk-benefit analysis of the likelihood of an individual developing an autoimmune disease, before it happens. Then you actually start to treat them with this kind of desensitising approach.”
The immune system is so central to our health, that there are many more areas where immunotherapy might be used in medicine further down the line. All inflammation is caused by the body’s immune response, and is often central to common conditions such as arthritis, stroke and bowel disorders. Some even believe disorders such as depression could be caused by inflammation, or that ageing is simply a problem of the immune system. These may well be the next frontiers in immunotherapy.
Sadly, the biggest barrier preventing immunotherapies from becoming commonplace in medicine might be the cost. The most effective treatments involve removing, testing and modifying individual patients’ own tissues and cells, a very different model to traditional therapies based on chemicals that could be mass-produced. Some experts believe this is not something health systems can afford to do.
“It’s an existential crisis for medicine, a great problem looming for society,” says Dan Davis, professor of immunology at Manchester University and author of The Beautiful Cure: Harnessing Your Body’s Natural Defences. “The more complicated and expensive the treatment, the harder it will become to reduce health inequalities and have an affordable care system.”
How cancer vaccines work
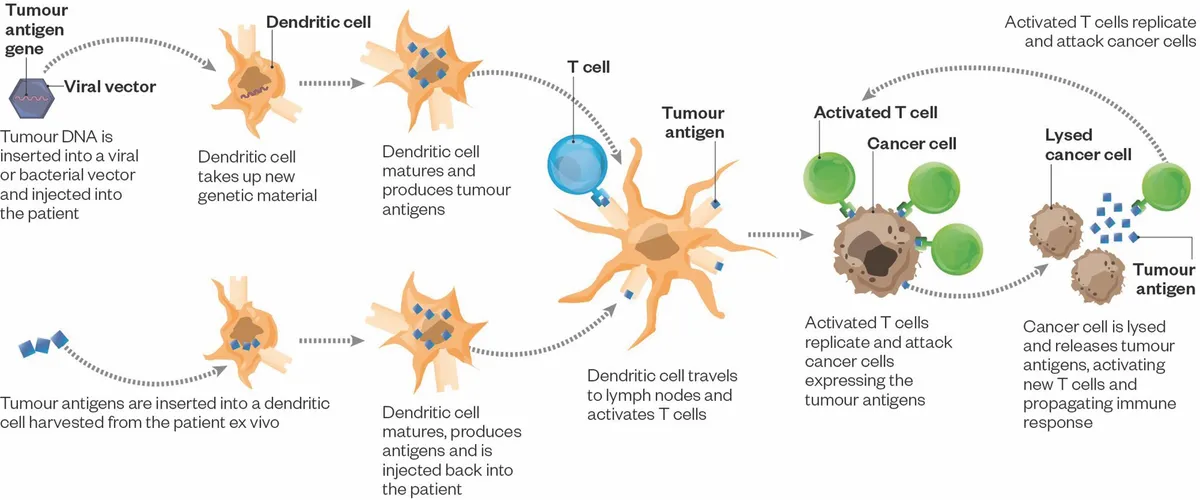
Cancer vaccines aim to ‘teach’ the immune system to attack and break down tumours. First, a molecule must be found that is expressed exclusively by the cancer cells, known as an antigen. Then the body’s immune cells must be encouraged to attack any cell found to have that antigen.
Immune cells known as dendritic cells are key to cancer vaccines, as they ‘present’ antigens of interest to the other immune cells, effectively telling them which antigens to find and attack. Dendritic cells can be ‘educated’ to recognise tumour-specific molecules, either within the body or by growing them with the tumour molecules in the lab.
The key is finding the right tumour molecule, and presenting it in a way that makes it interesting to the immune system. The matured dendritic cells then present the key molecule to the immune system, stimulating T-cells to attack the tumour. Substances to boost the activity of the immune systems, known as checkpoint inhibitors, prevent the tumour from releasing signals that block immune system activity.

Follow Science Focus onTwitter,Facebook, Instagramand Flipboard
