Trials of an exciting new way of treating dangerous drug-resistant infections will start to report results in 2021. These treatments involve patients being injected with billions of virus particles.
What are bacteriophages?
'Bacteriophages' are simply viruses that infect and kill bacteria.
Such viruses – also known as just ‘phages’ – are found everywhere there are bacteria and replicate by inserting their genes into bacterial cells. Once infected with the virus genes, the bacterial cell goes awry and starts producing viral proteins, which assemble into new viruses.
Within as little as half an hour, the cell bursts and tens or hundreds of new viruses are released to repeat the cycle in another host. They’re remarkably effective at this: some studies suggest that for every grain of sand on Earth there are over a trillion phages in existence at any one time.
Given their natural bacteria-killing abilities, these nanoscopic predators could be key in the fight against the growing problem of antimicrobial resistance. And the idea of using them in medicine isn’t really new at all.
Read more of the ideas you need to know in 2021:
- Beyond DNA: How proteins let us get up close and personal to our ancient relatives
- Phobias, paranoia and PTSD: Why virtual reality therapy is the frontier of mental health treatment
- The cracks in cosmology: Why our Universe doesn't add up
- Rewilding: Can it save our wildlife and temper climate change?
- How swarm spacecraft could help us understand Earth like never before
- Rise of the Clones: 7 ways cloning is already happening
Félix d’Hérelle and the discovery of bacteriophages
Over 100 years ago, in 1919, an eccentric scientist called Félix d’Hérelle breezed into a children’s hospital in Paris and told doctors that he had found a way to treat dysentery (an infection of the intestines). This was decades before antibiotics such as penicillin were available, when common bacterial infections killed millions of people a year.
While studying colonies of bacteria, d’Hérelle realised that some kind of disease was spreading through his bacterial plates. Although barely anything was known about viruses at the time, d’Hérelle came to the extraordinary conclusion that he had found a virus that preyed on bacteria, not humans – a microbe of a microbe. He named the virus a bacteriophage (‘bacteria-eater’) and began using it to kill off bacterial infections in animals.
This was long before the existence of regulators like the Federal Drug Administration (FDA) in the US or the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, who rigorously test new drugs and therapies before they’re approved for use in patients.

So to prove his experimental treatment was safe to use on children with dysentery, d’Hérelle simply drank a vial of the phages in front of the hospital doctors. The doctors agreed that if he was alive the next day, they’d try the treatment at the hospital.
The next day, d’Hérelle was fine (he’d already tested the phages on himself and his family). When several desperately ill children arrived suffering from dysentery, they were each given a vial of the pure bacterial viruses to drink.
The treatment was a success. The children quickly recovered and left hospital within days. His phages were soon being touted as the latest wonder drug and by the 1930s were being shipped around the world to treat all manner of different bacterial infections.
Why were bacteriophages forgotten?
How come then, in 2020, so few people have heard of phage therapy? And why, given that the world is so desperate for new antibiotics, are we not using it in hospitals all the time? It’s a long story, but by the 1950s the Western world had largely forgotten all about phages.
Drugs such as penicillin had come onto the market, and were far cheaper and easier to mass produce than live viruses. In most parts of the world, medical research focused on developing new classes of antibiotics and the idea of using viruses to treat infections was gradually abandoned.
The only countries that still used phages to treat infections were Soviet countries, where there was a shortage of drugs like penicillin. But the Cold War prevented any communication between Soviet scientists and the rest of the world, so their improvements in using phages went unnoticed. By the 1980s, Western medicine had almost entirely forgotten phage therapy had ever existed.
Now, as drug-resistant bacteria becomes a bigger and bigger problem in hospitals around the world, the idea of using viruses is attracting attention once again. Desperate patients in the US and Europe now travel to ‘phage clinics’ in former Soviet countries, such as Georgia, to try and cure infections that are no longer treatable with antibiotics.
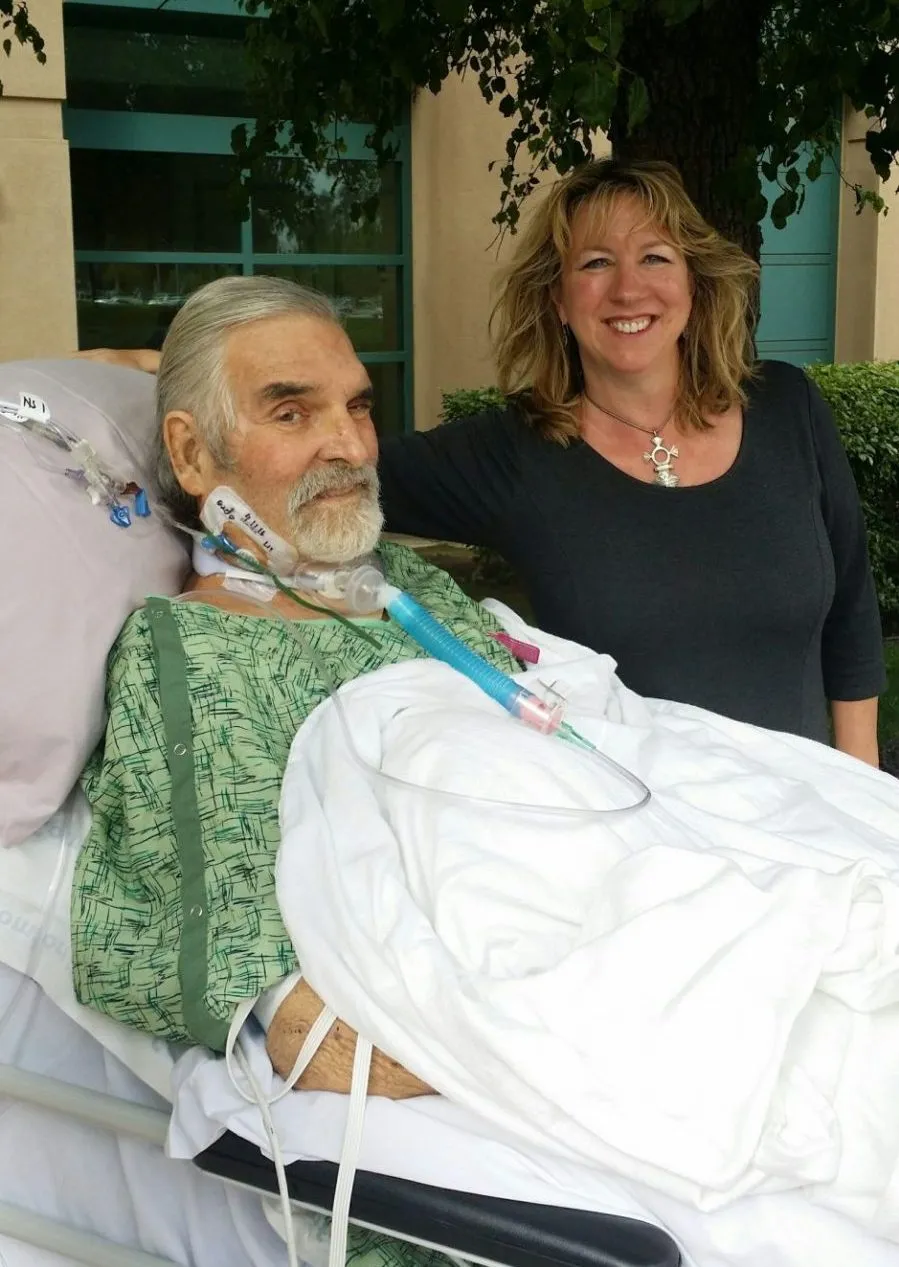
Despite the renewed interest, outside these regions phage therapy has only been used successfully in a few experimental cases. In 2015, scientist Tom Patterson fell ill with pancreatitis while on holiday in Egypt. The cause turned out to be a multiple-drug-resistant strain of bacteria.
With doctors unable to control the infection, Tom quickly fell into a coma. Almost a year of conventional treatments failed, but as a last resort, Tom’s wife Steffanie Strathdee, an HIV researcher, decided to do her own research and stumbled upon the idea of using phages to kill the bacteria that was killing her husband.
She spent months trying to connect Tom’s doctors with scientists all over the world who might be able to source the right phages to control the infection (as well as completing the piles of paperwork required to gain legal approval for a treatment that doctors effectively had to make up as they went along).
Thanks to injections of an experimental cocktail of phages – sourced from Russia, the US Navy and raw sewage – Tom was eventually brought back from the brink of death.
How are bacteriophages used?
Phages are not easy to work with. Every different strain of bacteria – of which there are millions – has a particular strain of phage that preys on it. So every patient needs to be treated with exactly the right strain of phage for the bacteria causing their infection.
The body also removes phages from the bloodstream quickly and, just like antibiotic drugs, bacteria can quickly develop a resistance to them. This is why a combination of phages often must be used to ensure that any bacteria with resistance to one phage are killed by another. This adds to the complexity of developing a treatment regime.
There’s also the fact that up to half of all phages are what are known as ‘temperate phages’. These viruses don’t always burst the bacterial cell open and kill it. Instead, they prefer to lie dormant in the cell’s genome or replicate slowly without killing their host. Scientists can get around this by genetically altering phages so that they use the more violent ‘bursting’ strategy, rather than lurking.
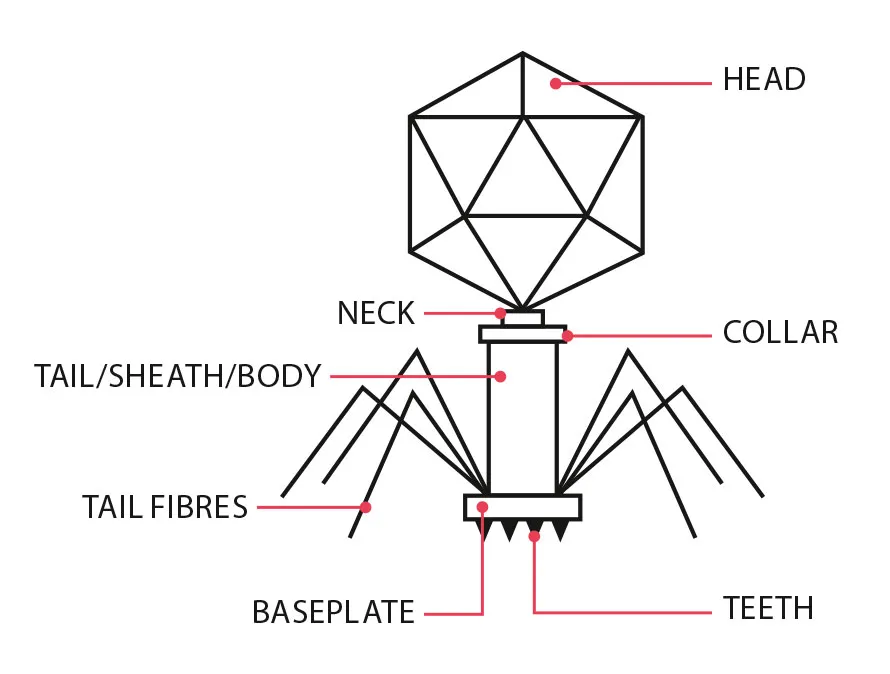
In 2018, at London’s Great Ormond Street Hospital, a cocktail of three genetically engineered phages were used to treat a young patient who had suffered from breakouts of multiple-drug-resistant infections throughout her body for years.
The teenage patient had undergone a double lung transplant due to cystic fibrosis, a condition that causes a build-up of mucus in the lungs. Soon after the procedure, despite several courses of antibiotics, infections spread from her lungs to her liver and, eventually, there were even pockets of bacteria pushing up through the skin on her arms, legs and buttocks.
Again, the development of a phage-based treatment required an international collaboration of doctors and phage researchers to find, purify and engineer the right phages for the particular strain of bacteria in the patient – as well as the laborious bureaucratic hurdles required to give an entirely new and unregulated medical treatment to a child. But it was a success – the treatment brought the infection under control and saved the girl’s life.
Phage libraries
These success stories have helped accelerate efforts to develop phage therapy in the US and Europe, with similar good news from experimental treatments and trials in animals in other countries. Yet despite these positive examples, there are still major obstacles to phage therapies becoming cost-effective and reliable mainstream treatments.
The main obstacle is that every patient is likely to be infected with a unique mix of bacterial strains, so every treatment must be bespoke. This means drug manufacturers can’t mass-produce doses and it has also made it impossible for drug regulators to approve phage therapies: every case is likely to involve a completely different mix of viruses.
Thankfully, collaborative efforts between researchers, public health bodies and regulators are helping to overcome these hurdles. The idea is that by using modern DNA-sequencing technology, enormous libraries of phages could be quickly scanned to find a suitable match. Matches could then also be screened to ensure they don’t contain any genes that might cause toxicity in the patient.
Drug regulators would assess the safety of the library as a whole, the methods of purifying the phages and the method of administration, rather than trying to assess every individual treatment.
When a patient had a drug-resistant infection, information on the exact strain of bacteria would be sent to a phage library, which would be scanned for suitable matches. The matches would then be sent out in a vial to the hospital where the patient was being treated. With this method, a lifesaving and bespoke cocktail of viruses could be produced in a matter of days.
That’s the plan, at least. Several clinical trials are underway that hope to prove, once and for all, that phages can be a reliable method of treating infections, and the collaborative efforts of authorities, scientists and doctors continue in the hope of setting up regulatory systems that can green-light bespoke phage therapies in a cost-effective way.
Hopefully none of us will ever face the terrifying prospect of an infection with bacteria that are resistant to conventional antibiotics. But if we do, it’s good to know that these ancient bacterial killers can be called upon to help us out.
Read more about viruses:
Life cycle of a bacteriophage
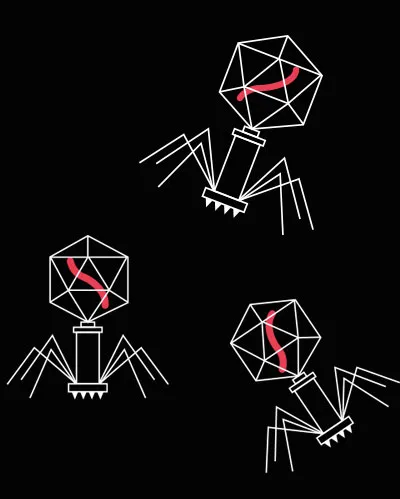
Free-floating phages drift around in the environment, hoping to encounter a bacterial host.
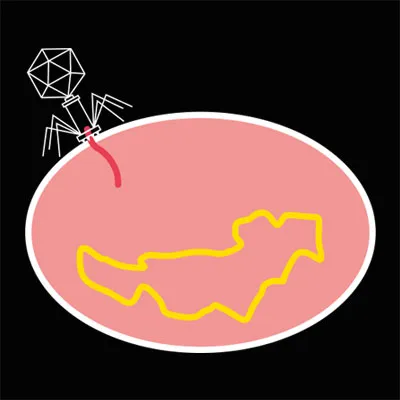
Once the phage binds to a suitable host, it injects its genetic material into the cell.
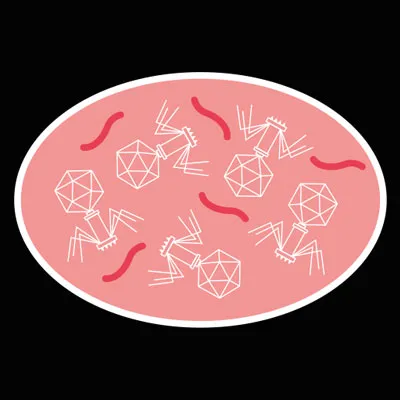
If the phage’s genetic material evades the cell’s defences, the cell will start manufacturing the proteins encoded in the virus genome.
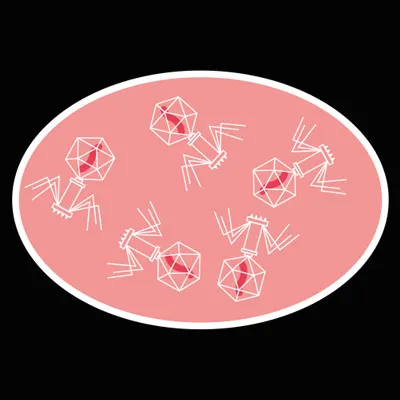
This causes a build-up of viral proteins inside the cell, which assemble into hundreds of new bacteriophages.
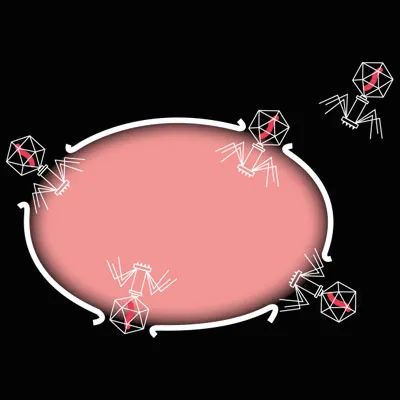
The phage eventually instructs the cell to produce a protein that bursts it open. The bacterium is dead and the new phages are released to repeat the cycle in another host cell.
- This article first appeared inissue 358ofBBC Science Focus Magazine–find out how to subscribe here
