Often described as non-enzymatic browning, the Maillard reaction gives a wide range of cooked foods their appealing flavours and colours. Sped along by heat, the Maillard reaction is actually a series of reactions,startingwith one between protein and a reducing sugar,such as glucose or fructose. The reactions produce flavour chemicals and browning in foods including fried onions, toast, steak and roasted coffee.
French chemist LouisCamille Maillard first reported the reaction between proteins and sugars in 1912. In recent decades, scientists have unearthed more of Maillard’s detailed mechanisms.
When heat hits food, sugars react with the amino acids that make up proteins to form glycosylamine. This unstable chemical rearranges to create a ketosamine, setting off a cascade of further reactions to produce hundreds of new substances, some of which contribute to flavour and aroma.
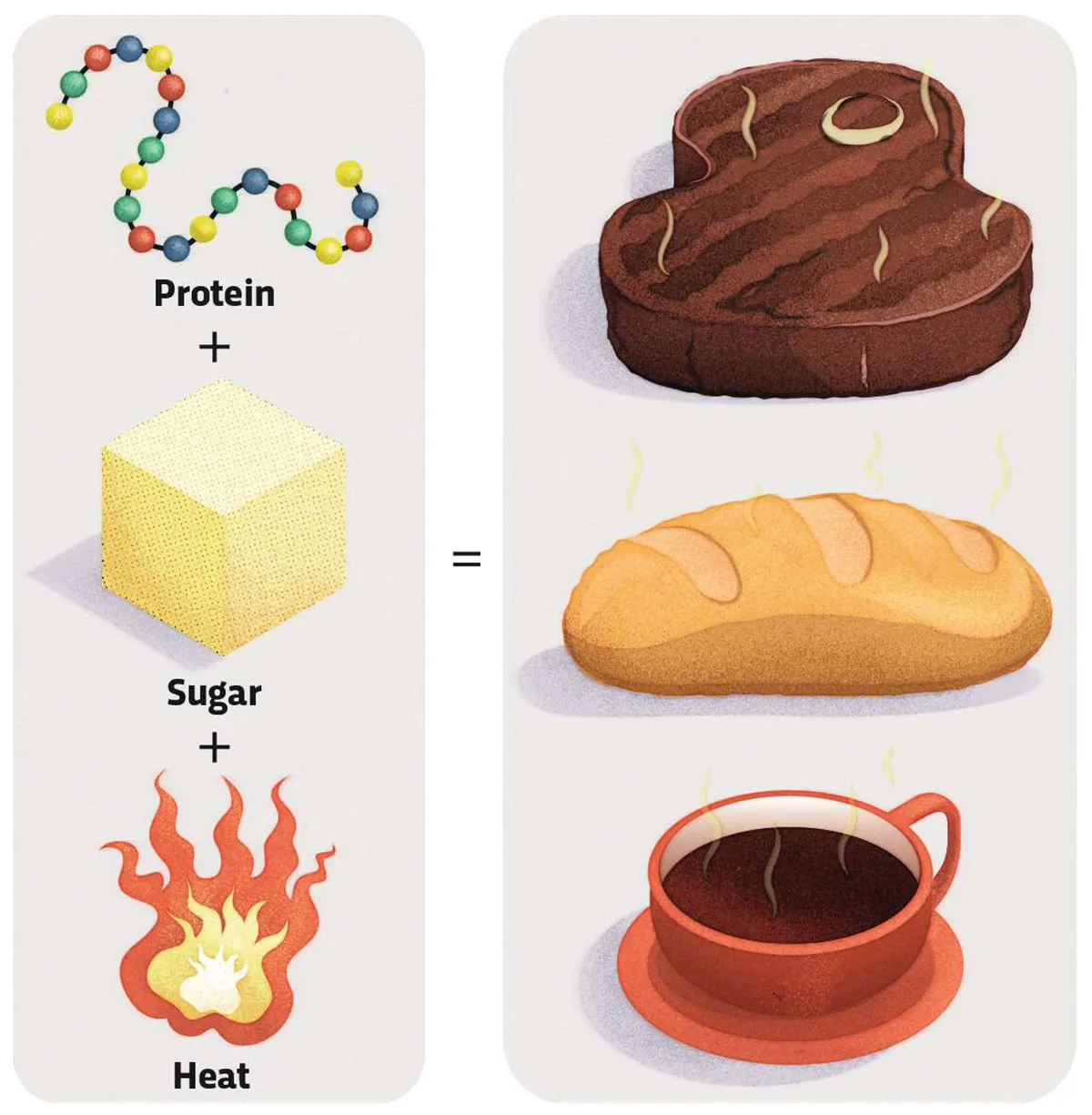
These include pyrazines, which have a toasted flavour,along withmeaty furans and sweet furanones. Finally, the reaction creates large polymer molecules called melanoidins,which give a brown colour.
In order to obtain Maillard products in a short time, the temperature should exceed 100ºC, with an ideal range between 110ºC and 170ºC. If the temperature is too high, then bitter flavours can develop.
A downside of the Maillard reaction is that it creates a carcinogen called acrylamide as a by-product. Levels of this increase with longer cooking times.
Read more:
- Is ‘snakebite’ just a mixture of lager and cider, or a chemical reaction between the two?
- When I make chocolate chip cookies, why don’t the chocolate chips melt in the oven?
- How do you thicken a sauce?
- What is the yeast doing inside my bread?
Asked by: David Walker, via email
To submit your questions email us at questions@sciencefocus.com (don't forget to include your name and location)
