The coronavirus SARS-CoV-2 that has dominated the news since early 2020 has something in common with other diseases that have hit the headlines in recent years. SARS-CoV-2, just like Ebola, HIV and MERS before it, originated in wildlife before ‘spilling over’ into humans.
SARS-CoV-2 currently appears to have originated in horseshoe bats and was potentially transferred to humans via an unknown species, possibly pangolins. But other so-called zoonotic diseases (illnesses that spread from animals to humans, and vice versa) originated in the likes of chimps, camels and mice.
While the existence of zoonotic diseases has been known for decades, the coronavirus pandemic has brought into sharp focus how closely our health is connected to the health of the animal species with which we come into contact.
“This pandemic is just a tragic wake-up call,” says epidemiologist Dr Jonna Mazet at the University of California, Davis. Mazet was principal investigator on the PREDICT project, a $207m (£150m approx) global effort run from the US to build a clearer picture of the viruses lurking in wild animals that could spill over into humans and wreak havoc.
From 2009 to 2019, the project’s scientists collected samples of animal blood, saliva and dung from fields and forests in 30 countries. They found 940 virus species that hadn’t been previously identified, including 160 coronaviruses and one new Ebola virus that were previously unknown.
But this may just be the tip of the iceberg. “We estimate there are probably about 500,000 viruses that could infect people that have not been characterised or detected by science,” says Mazet.
Not all of these would cause disease, but it shows the scale of the problem. Combine the number of viruses capable of spilling over into humans with deforestation, bushmeat hunting, and farming activities encroaching into wildlife-rich areas, and it’s a dangerous cocktail.
It’s no wonder then that the US Centers for Disease Control and Prevention (CDC) estimates that 75 per cent of the new or emerging infectious diseases we are contending with originated in animals.
Too close for comfort
A clear demonstration of the close connection between human and animal health came in June 2020, during the first months of the pandemic.
Danish authorities reported that SARS-CoV-2 had leapt from humans to mink and spread extensively on mink farms. Not only that, but the virus also jumped back to humans, causing a spike in the country’s coronavirus cases.
What was particularly worrying was that some of those who caught coronavirus from mink had a new virus variant.
Thankfully, tests showed that the variant’s mutations did not make it more contagious or deadly. SARS-CoV-2 has also been detected in a raft of other species that humans come into contact with, including gorillas at San Diego Zoo Safari Park and a snow leopard at Louisville Zoo.
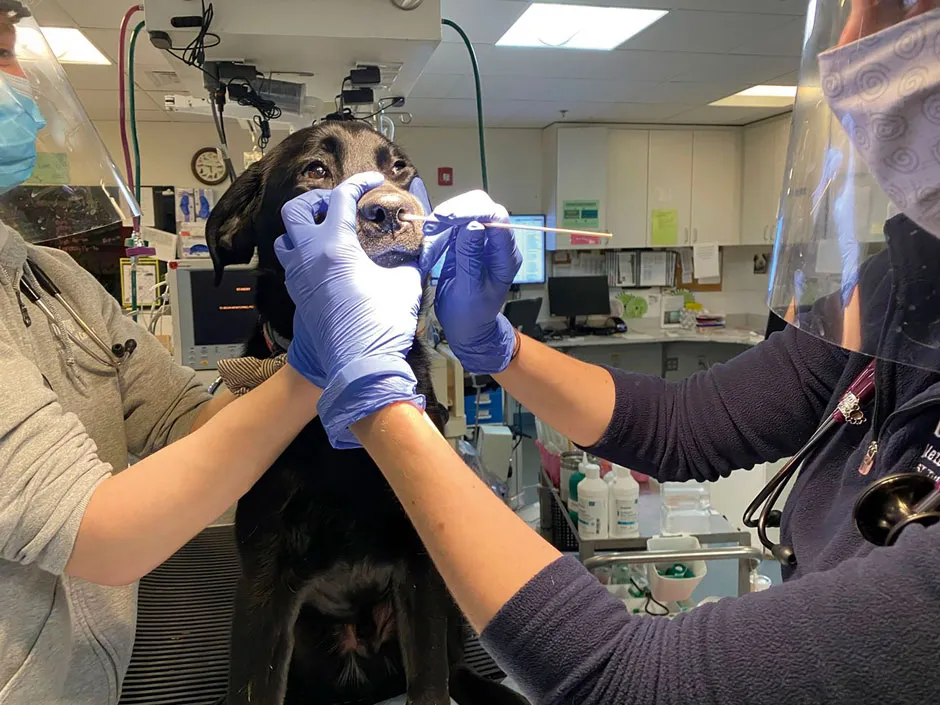
Dr Kaitlin Sawatzki, a postdoctoral researcher at Tufts University in Massachusetts, has been testing pets brought into a veterinary clinic to see whether any of them picked up SARS-CoV-2 from their owners.
While Sawatzki is reticent to say how many of the dogs and cats tested positive for coronavirus antibodies before the research is published, she says that what she found was “comparable” with an Italian study of pets from 2020 in which 3 per cent of dogs and nearly 6 per cent of cats tested positive.
The good news is that dogs don’t appear to get ill with SARS-CoV-2, and for cats it may only cause a mild illness.
“As far as domestic pets go, I don’t think those are going to play any real role in a public health concern,” says Sawatzki. “But if we’re thinking of the bigger picture here, other than the gorillas, all of the animals that we’ve seen these accidental transmissions in have been carnivores.
"So I’m concerned about wild carnivores and the potential for them to be infected and then for reverse transmission back into humans.”
A new vaccine?
It’s for situations like this that The Vaccine Group (TVG), a spin-out company at the University of Plymouth, is currently developing a new SARS-CoV-2 vaccine that could be injected into animals as well as humans.
The vaccine uses a benign virus, bovine herpesvirus 4, that acts as a carrier for genetic material from SARS-CoV-2.
What makes bovine herpesvirus 4 particularly useful as a virus vector is that it can harmlessly infect many different animal species, making it a flexible vaccine that could be injected quickly if an outbreak cropped up in a new animal species that humans come into close contact with.
It is being prepared for the unexpected that’s important. “We can get a feel for where SARS-CoV-2 is going, but it can always surprise,” says Dr Michael Jarvis, associate professor in virology and immunology and TVG’s chief scientific officer.
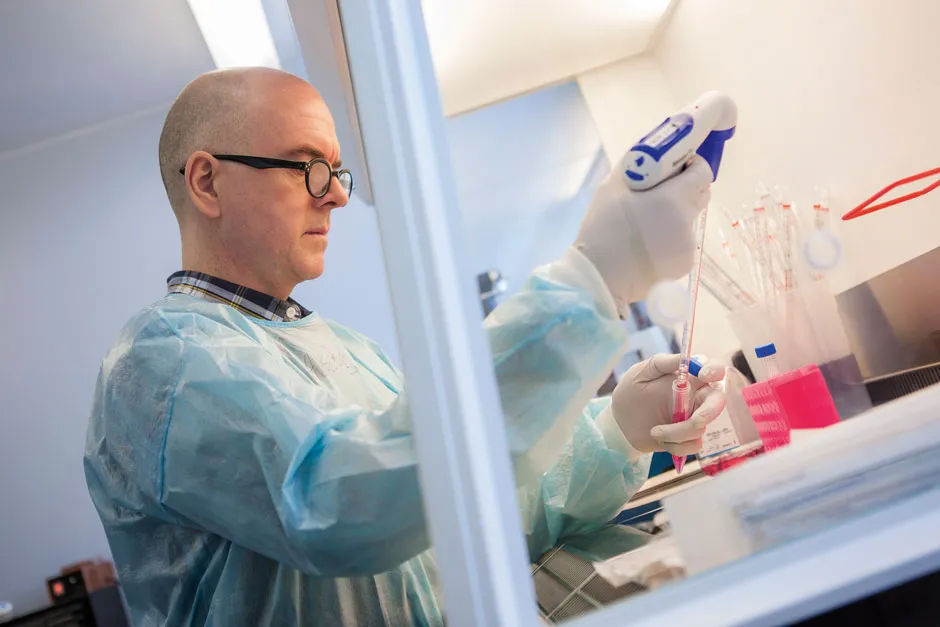
Rather than just carrying genetic material for the virus’s protein spike, like the current vaccines do, this new vaccine also contains genetic material from the shell that surrounds the virus’s genetic material as well as its outer membrane.
These parts of the virus don’t change between variants, so the vaccine can provide protection against them. If tests in pigs show the vaccine is effective in generating an immune response, it would soon be ready and waiting for another coronavirus outbreak in animals and, with further testing, could be used as a new variant-proof vaccine for humans.
Russia’s state veterinary service also announced that it has developed a SARS-CoV-2 vaccine for animals, but few details have been provided about what form it takes.
There’s a snag. Vaccinating wild animals to prevent ‘spillovers’ of viruses – whether it’s SARS-CoV-2 transferring back into humans, or a new coronavirus or a new Ebola – is a massive logistical challenge because of the sheer number of animals involved.
But what if rather than having to inject every single animal, the vaccine could spread itself?
It’s an idea that’s gathering momentum. “My impression is probably biased but interest in self-disseminating vaccines has grown exponentially over the last few years,” says Prof Scott Nuismer, an expert in self-disseminating vaccines at the University of Idaho.
Read more about vaccines:
- Everything you need to know about the UK's coronavirus vaccines
- Coronavirus vaccine: Is it safe?
- Why do some people experience more vaccine side effects than others?
One approach being explored is to have a ‘transferable vaccine’ that is painted onto the fur of a handful of bats, mice or any other species in a population. Then, when their neighbours lick them during grooming, they ingest the vaccine and become immune.
Such a vaccine that could provide vampire bats with immunity to rabies has already been trialled by researchers at the University of Glasgow.
“Technologically we’re almost there with transferable vaccines, the final challenge is just fine-tuning the delivery system in a way that encourages a mouse or fruit bat to lick their friends and ingest the vaccine,” says Nuismer.
The big drawback of transferable vaccines is that the vaccine pasted onto each bat or mouse will only spread so far. Far more effective is a ‘transmissible vaccine’ that could spread indefinitely.
Here a bat or a mouse, or whatever the virus reservoir species is, would be injected with the vaccine, passing it on to its neighbours by harmless infection, which then pass it on to its neighbours and so on.
Researchers at Jarvis’s lab at Plymouth are already on the case and are currently developing a transmissible Ebola vaccine for apes and bats, and another for Lassa virus in rats.
Risk management
While transmissible vaccines are further off, it’s worth thinking of the risks. For starters, what if the virus used in the vaccine mutates back into its original form capable of causing the disease?
The solution, says Nuismer, is to not use an attenuated vaccine in which the virus largely remains intact. A safer alternative is to use a recombinant vector – a benign virus that doesn’t cause disease and so can’t go rogue.
Into this, a small amount of genetic material from the disease-causing virus is added to stimulate an immune response.
“Then it bounces from individual to individual, transmitting that wild virus and it immunises as it goes,” says Nuismer.
“Everything I know as an evolutionary biologist suggests that this idea of putting this gene in and having it turn into some monster virus that kills us all seems extremely unlikely.”
This vector would need to be specific to the species it’s inoculating to prevent the vaccine spreading uncontrollably to different species – species in which no trials have been conducted to ensure it’s not harmful.
Then there’s one final risk – that eradicating one virus by vaccination would leave the door open to another that’s more dangerous.
“If you go into a wildlife population and you knock out one virus, do you create an empty niche that can be invaded by some other virus that wasn’t a problem before? We know this from non-viral ecology – if you destroy an area, you’re going to get invasive species,” says Nuismer.
To rule this out, a transmissible vaccine would need to be tested on small groups of animals to check that no new harmful viruses creep in. But before pre-emptive wildlife vaccination programmes can be an effective way to prevent the next pandemic, we need a much clearer view of the viruses lurking out there.
In recognition of the need to do more to combat zoonotic diseases, in September 2020 the US government agency USAID announced a new $100m (£73m approx) project, STOP Spillover, to try to detect spillover events more rapidly and develop new ways of containing them.
Even just looking at the coronaviruses, there’s a clear case for prioritising wildlife vaccines.
“MERS, SARS, SARS-CoV-2 haven’t been separated by much time in the grand scheme of things. I don’t see any reason to think there won’t be another one in the next 10 or 20 years,” says Nuismer.
But before the vaccines can be developed, we need to know more about which species coronaviruses live in and whether they bounce between different species.
“The first step is to get out there and figure out what are these things actually doing.”
About the author, Andy Ridgway
An award-winning journalist, Andy has spent nearly 20 years in the profession as is a past deputy editor of BBC Science Focus. Andy was also named Editorial Person of the Year in the BBC Magazines Awards for Excellence in 2010.
Read more about viruses:
