Russia’s Sputnik V coronavirus vaccine is 91.6 per cent effective against symptomatic COVID-19, interim trial results suggest.
No serious adverse events were deemed to be associated with vaccination, and most reported adverse events were mild, including flu-like symptoms, pain at the injection site and weakness or low energy.
Interim data from the Phase 3 trial of the coronavirus vaccine from Russia, Gam-COVID-Vac (Sputnik V), suggests a two-dose regimen of the vaccine offers 91.6 per cent efficacy.
The preliminary findings, published in The Lancet, are based on analysis of data from nearly 20,000 participants, three-quarters of whom received the vaccine and one quarter received a placebo.
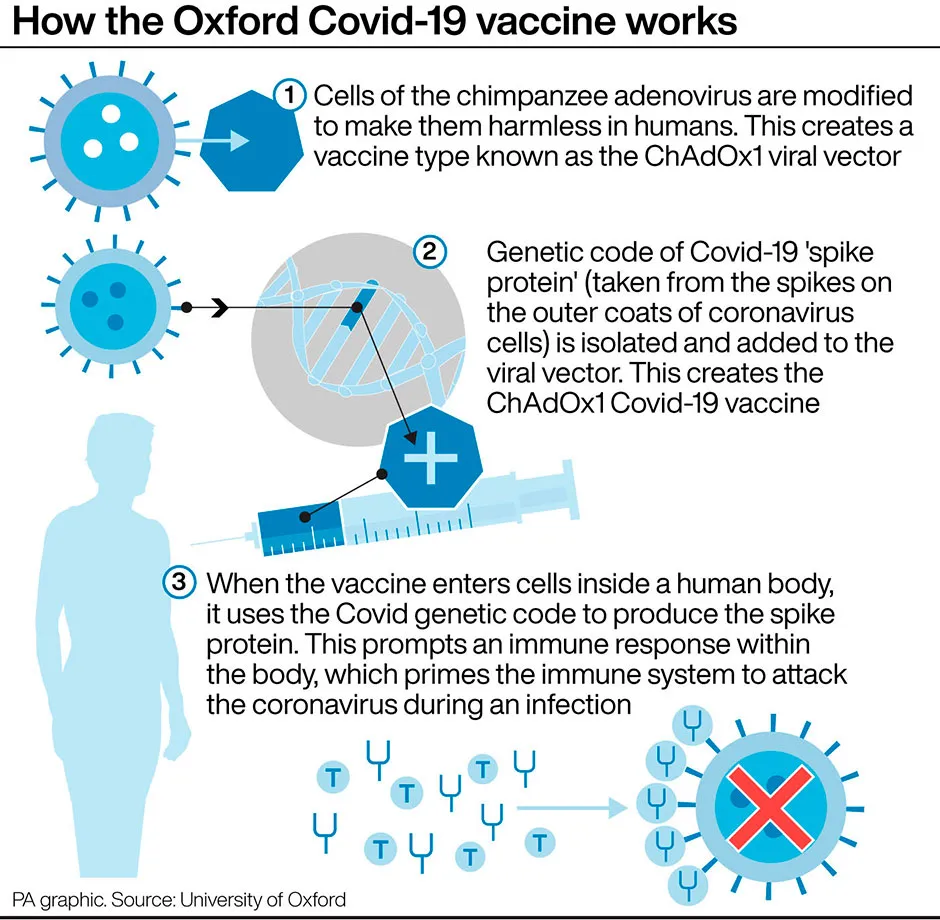
Like the Oxford vaccine, the jab is a two-part vaccine that includes two adenovirus vectors – genetically altered common cold viruses from chimpanzees – which have been modified to express the spike protein found on SARS-CoV-2, the virus that causes COVID-19.
Read more about coronavirus vaccines:
- COVID-19: Everything you need to know about the Janssen coronavirus vaccine
- COVID-19: Everything you need to know about the Novavax coronavirus vaccine
- COVID-19: Everything you need to know about the Valneva vaccine
The adenoviruses are also weakened so that they cannot replicate in human cells and cannot cause disease.
In the trial, participants were given the first vaccine dose, followed by a booster dose 21 days later.
According to the researchers, using a different adenovirus vector for the booster vaccination may help create a more powerful immune response, compared with using the same vector twice.They suggest this is because it minimises the risk of the immune system developing resistance to the initial vector.
“Our interim analysis of the randomised, controlled, Phase 3 trial of Gam-COVID-Vac in Russia has shown high efficacy, immunogenicity, and a good tolerability profile in participants aged 18 years or older," said Dr Inna V Dolzhikova, co-lead author, from the Gamaleya National Research Centre for Epidemiology and Microbiology in Russia.
Between 7 September and 24 November 2020 last year, a total of 21,977 adults were randomly assigned to receive the vaccine (16,501) or placebo (5,476).
In the vaccine group, 14,964 participants – and 4,902 in the placebo group – received two doses of the vaccine or placebo and were included in the primary interim efficacy analysis.
From 21 days after receiving the first dose – the day of the second dose – 16 cases of symptomatic COVID-19 were confirmed in the vaccine group, and 62 cases in the placebo group, equivalent to an efficacy of 91.6 per cent.
Researchers say the vaccine induced a robust antibody response and T-cell response, with data from 342 and 44 participants, respectively.Six of the 342 participants did not mount an immune response following vaccination, possibly due to older age or individual characteristics, according to the study.
The authors note that because COVID-19 cases were detected only when participants self-reported symptoms, followed by a test, the efficacy analysis only includes symptomatic cases of the virus.They say further research is needed to understand the efficacy of the vaccine on asymptomatic COVID-19, and transmission.
Read more about COVID-19:
- Ventilation and viral loads: the key misunderstandings of how coronavirus spreads
- Will COVID-19 be over by 2022?
The median follow-up was 48 days from the first dose, so the study cannot assess the full duration of protection.
Four deaths were recorded during the trial – three in the vaccine group, and one in the placebo group. In the vaccine group, one death was associated with a fracture, and two had underlying conditions and developed symptoms of the virus four to five days after the first dose of the vaccine.Both participants were deemed to have already been infected before inclusion in the trial.
In the placebo group, the death was associated with a stroke. None of the deaths were deemed to be associated with vaccination.
The trial included 2,144 participants more than 60 years old, and vaccine efficacy was 91.8 per cent in this group.
How do scientists develop vaccines for new viruses?
Vaccines work by fooling our bodies into thinking that we’ve been infected by a virus. Our body mounts an immune response, and builds a memory of that virus which will enable us to fight it in the future.
Viruses and the immune system interact in complex ways, so there are many different approaches to developing an effective vaccine. The two most common types are inactivated vaccines (which use harmless viruses that have been ‘killed’, but which still activate the immune system), and attenuated vaccines (which use live viruses that have been modified so that they trigger an immune response without causing us harm).
A more recent development is recombinant vaccines, which involve genetically engineering a less harmful virus so that it includes a small part of the target virus. Our body launches an immune response to the carrier virus, but also to the target virus.
Over the past few years, this approach has been used to develop a vaccine (called rVSV-ZEBOV) against the Ebola virus. It consists of a vesicular stomatitis animal virus (which causes flu-like symptoms in humans), engineered to have an outer protein of the Zaire strain of Ebola.
Vaccines go through a huge amount of testing to check that they are safe and effective, whether there are any side effects, and what dosage levels are suitable. It usually takes years before a vaccine is commercially available.
Sometimes this is too long, and the new Ebola vaccine is being administered under ‘compassionate use’ terms: it has yet to complete all its formal testing and paperwork, but has been shown to be safe and effective. Something similar may be possible if one of the many groups around the world working on a vaccine for the new strain of coronavirus (SARS-CoV-2) is successful.
Read more:
