Prof David Gracias is the director of graduate studies in the Department of Chemical and Biomolecular Engineering at Johns Hopkins University. He spoke to BBC Science Focus commissioning editor Jason Goodyer about his latest work on bioinspired microdevices that can release drugs directly into patients' gastrointestinal systems.
You based the technology on parasitic hookworms. Where did that idea come from?
We’ve been trying to deliver drugs through the gastrointestinal tract, and that is a formidable challenge because the gastrointestinal tract has a mucosa – a mucus layer. It’s kind of like a conveyor belt, it’s constantly moving. It moves and it sheds [cells] in different parts at different rates.
We are used to using a patch for controlled release on the outside of the body, but if you put a similar patch on the inside it won’t be retained. So, we were thinking, well, how does nature solve this problem? And we knew that there are worms and other organisms that colonise and live in the gastrointestinal tract for a long period. So, we started looking into them. They dig into the mucosa, so we had this new idea of doing that.
How big are the theragrippers and what are they made of?
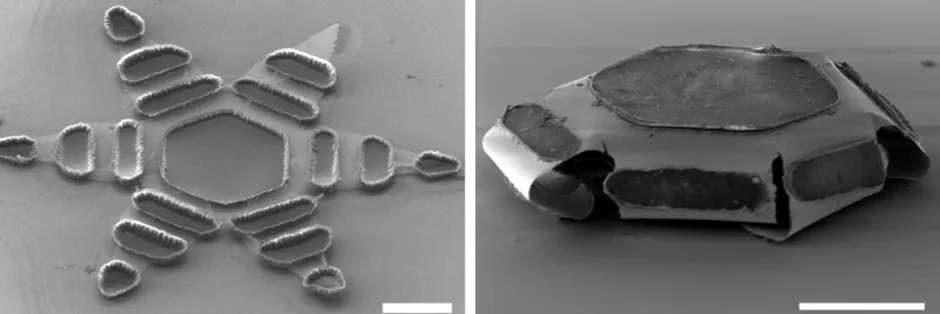
They are about a quarter of a millimetre, so barely visible to the human eye. And they are made out of metals and polymers.
Read more about drugs:
- Six drugs discovered by accident
- Magic mushrooms and mental health: Could psychedelic drugs treat depression?
- More than 1,700 deaths due to 'medication errors' in England every year
How did you choose their size?
We often optimise it, and it is dependent on the specific application. Larger sizes could potentially store more drug, but they become more invasive. People have made centimetre-scale devices, huge ones. But there’s a risk of blockages and other issues, so smaller is less intrusive, in our opinion. But then we need more of them to achieve a specific quantity of drugs to be delivered.
How do you go about manufacturing something so tiny?
We use similar processes to the microchip industry, so they are made on silicon wafer substrates. They are made using a process called photolithography, which is a standard workhorse in micro fabrication. And by doing so, we can make thousands of them at the same time on a wafer, which is what makes it cost effective.
Once a patient takes them, how do the theragrippers release the drug?
They can be taken either orally or through enema. The principle on which they work is like a loaded spring. Or you can think of it as a mousetrap. So, rather than a coiled spring, there are thin films that are under stress and they want to release. But the idea is the same.
We constrain them by using what we call a polymer trigger, and we work with different polymer triggers to make them so we can tune the polymer trigger to the environment around the gripper. So, for example, in this present paper, we use a temperature-responsive trigger. We refrigerate them and then, because they are cold, the temperature trigger keeps them flat. In our animal tests, when they enter the body, they thermally equilibrate with the body, which is at a higher temperature of about 37°C or so, and that triggers them. In principle, you could trigger them by many different specific environments in the body, for example, pH or biomolecules.
Once they trigger, how do they attach to the mucosa?
The theragrippers have claws and the claws release significant force and they attach. The drug is loaded on a patch [held inside] and it diffuses.
Why is it so important to have systems that are slow release?
There are many motivating factors for sustained-release drug delivery. One of them is compliance. At the start of the paper, we talk about the annual waste of $600bn due to imperfect adherence to treatment. What that means is that if you get a condition and have to take medication, you may forget to take it or you may miss taking it, so compliance and adherence is hard and so that is one motivating factor. If you have a system that is continually releasing a drug then you don’t need to take many doses.
The second one is that sustained release can maintain a uniform level of drug rather than having a lot of spikes, every time you take a pill, you get a jump, for example.
And the last one is that we live in a world where we want convenience and we want to not worry about these things. You can see that in products like the nicotine patch, for example, people just put it on and then they can forget about it for the rest of the day.
What type of conditions could theragrippers be used to treat?
We are looking at a variety of applications. In our present demonstration, we used a painkiller, but we are looking at other drugs.
What is the current stage and where you would like to go next?
There’s a lot of excitement in this new area of dynamic, smart machines and robotics. I’m definitely excited to be part of this vision for the future of medicine, which is this idea of advanced therapeutics. We coined this term ‘active matter therapeutics’, [to describe this new style of medicine] and I think that is going to be the dominant paradigm for the next few decades as we make medicine more efficient, safer, and more effective.
There are two avenues that we are trying to advance. One is the engineering side – we are looking at using capsules and putting them in other parts of the body. What sizes can we use? What drugs can we load? There’s a lot of engineering research that we’re planning. On the clinical side we would like to eventually advance this to humans. It’s a big leap to go from the laboratory to the animal, which we did, but it’s another leap to go from the animal to the human, mainly because of safety considerations.
My collaborator, Florin Selaru, is a practising gastroenterologist so this is a great partnership because I’m primarily coming from the engineering side but he’s a medical doctor and actually does a lot of procedures with patients.