Up to 30 million doses of a COVID-19 vaccine could be available to the UK by September if the vaccine candidate developed by Oxford University proves successful in human trials, the government has said.
Business Secretary Alok Sharma said the first clinical trials were “progressing well” and that all phase one volunteers had received their vaccine dose “on schedule earlier this week”.
Researchers at the Oxford Vaccine Group began testing the vaccine candidate, called ChAdOx1 nCoV-19, in humans last month, 24 April, to see whether it can protect healthy people from COVID-19.
Read more about coronavirus vaccine development:
- Oxford coronavirus vaccine ‘significantly reduces viral load’ in monkeys
- How are scientists developing a coronavirus vaccine?
- Coronavirus vaccine: could genetic material help defeat COVID-19?
The programme will allow researchers to assess the safety of the candidate, and its ability to generate an immune response.
The vaccine will be given to half the participants, while the remaining volunteers are given a licensed meningitis vaccine to be used as a control.
When analysing results, statisticians will compare the number of infections in the control group with the number of infections in the vaccinated group. Therefore, it is necessary for a small number of study participants to develop COVID-19.
In the coronavirus daily briefing on 17 May, the government announced its pledge to invest an extra £84m into the University of Oxford's search for a vaccine. Sharma also said £38m will be put towards building a "rapid deployment facility" to ensure any successful vaccine is made widely-available.
There are various vaccine candidates being trialled currently. In the UK, Imperial College London is also progressing with its vaccine candidate and will look to move into clinical trials by mid-June, with larger scale trials in October.
A thousand healthy volunteers involved in the trial
For the Oxford Vaccine Group testing, up to 1,102 participants have been recruited across multiple study sites in Oxford, Southampton, London and Bristol.
Volunteers must be aged 18-55, and cannot have tested positive for COVID-19.
They must also be in good health, not be pregnant or breastfeeding, and must not have previously taken part in a trial with an adenoviral vaccine or received any other coronavirus vaccines.
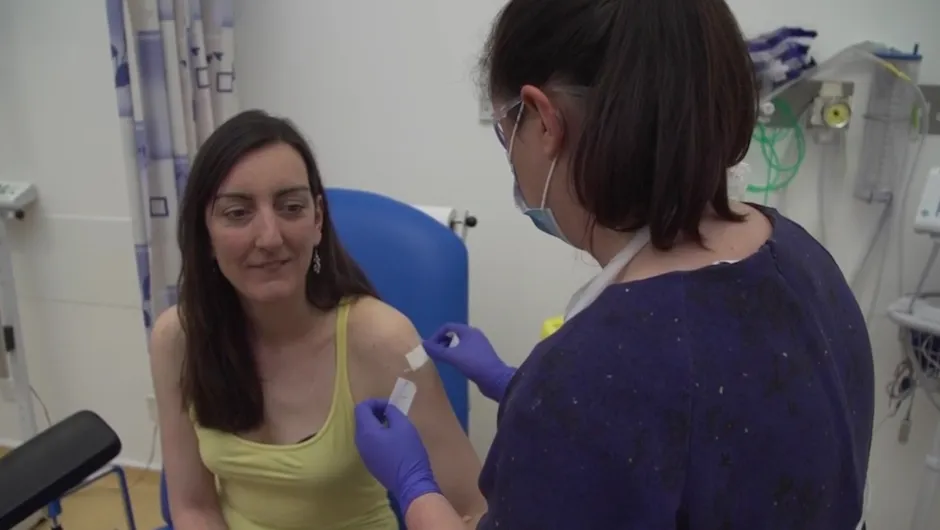
Vaccinations staggered over the first five days
The volunteers have been randomly allocated to receive either the ChAdOx1 nCoV-19 vaccine or a licensed meningitis vaccine that will be used as a control for comparison.
The dose used in the trial was chosen based on previous experiences with other ChAdOx1-based vaccines.
Participants will not know whether they have received the COVID-19 vaccine or the control vaccine until the end of the trial.
On April 24, the first two participants were vaccinated, one with the ChAdOx1 nCoV-19 vaccine and one with the control vaccine.On day three, six more volunteers were vaccinated, half with the COVID-19 vaccine and three with the control vaccine.On the fifth day, researchers began vaccinating larger numbers of participants.
Need a break from coronavirus news? Read some feel-good stories:
- Restoring sight to people who are blind, heat-resistant corals, and archaeology under lockdown
- Juggling otters, the benefits of gardening and tantruming toddlers
- Hangover cures, coal-free energy and beetles that help with hay fever
Symptoms tracked with a diary and blood tests
The participants were given an e-diary to record any symptoms they experience in the seven days after receiving the vaccine, and if they feel unwell for the following three weeks.
During a series of follow-up visits, blood samples will be taken to assess their immune response, and their observations checked.
If they develop COVID-19 symptoms during the study, they can contact a member of the clinical team, who will check if they have become infected with the virus.
If a participant becomes very unwell they will be reviewed by staff at the hospital.
Results depend on virus transmission rate
Statisticians will compare the number of infections in the control group with the number of infections in the vaccinated group.
Therefore, it is necessary for a small number of study participants to develop COVID-19.
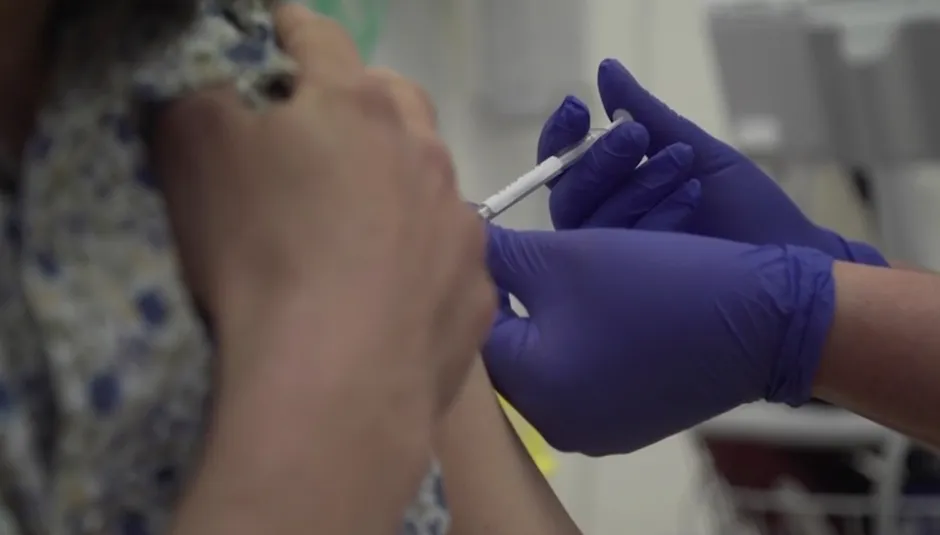
How quickly researchers reach the numbers required depends on the levels of virus transmission in the community.
If transmission remains high, enough data may be available in a couple of months but if transmission levels drop, this could take up to six months.
What is the Oxford vaccine candidate made of?
The vaccine candidate ChAdOx1 nCoV-19 is made from a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees, called ChAdOx1.
The adenovirus has been genetically changed so it is impossible for it to grow in humans.
Coronaviruses, such as the one that causes COVID-19, have an outer coat of protein spikes– recognisable in images of the virus. These spikes are what attach to the proteins on human respiratory cells, enabling the virus to enter and infect them.
The researchers vaccine candidate contains the genetic code of the protein spikes found on the coronavirus. They hope this will make the body recognise and develop an immune response to the spike protein that will help stop COVID-19 from entering human cells and therefore prevent infection.
Read more:
