From that probiotic yoghurt your mum swears by, to the growing number of Instagram gut health gurus, many have touted the role bacteria can have in boosting your belly’s microbiome. However, little is said about the balance of your intestinal fungi, which could have as much impact on your health.
At least what’s been shown by an intriguing new study from the University of Utah Health.
Experimenting with mice, researchers have shown that while fungi can thrive in a healthy gut, they may also cause intestinal damage leading to inflammatory bowel disease (IBD) similar to Crohn's disease.
To reach this finding, the researchers analysed the makeup of the yeast Candida albicans – one of the main species of fungi that are found in the human gut – in the intentional systems of mice.
They discovered that although the mice populated with the yeast in its normal state were healthy, mice intestinal systems filled withCandida cells(known as hyphae) experienced damage resembling IBD.
However, the researchers also found that the immune systems of healthy mice targeted the fungi when it switched to a harmful state, inhibiting disease from forming.
"The immune system is constraining Candida to its least pathogenic form," explained Dr Kyla Ost, the study's lead author. "It shows us that the communication between host and microbe can be friendly, as opposed to antagonistic.”
Read more about the microbiome:
- Should we be paying more attention to our poo?
- Psychobiotics: your gut and mental health
- The British microbiome: how our guts can tell us more than our genes
But that’s not all. The researchers also theorised that a vaccine could be developed in future to protect patients against inflammatory bowel disease.
Mice prone to an IBD-like condition were found to be less likely to develop the disease when administered with a vaccine currently designed as a remedy for vaginal yeast infections.
The study scientists are now working on a vaccine that could mitigate IBD in people and improve their gastrointestinal health.
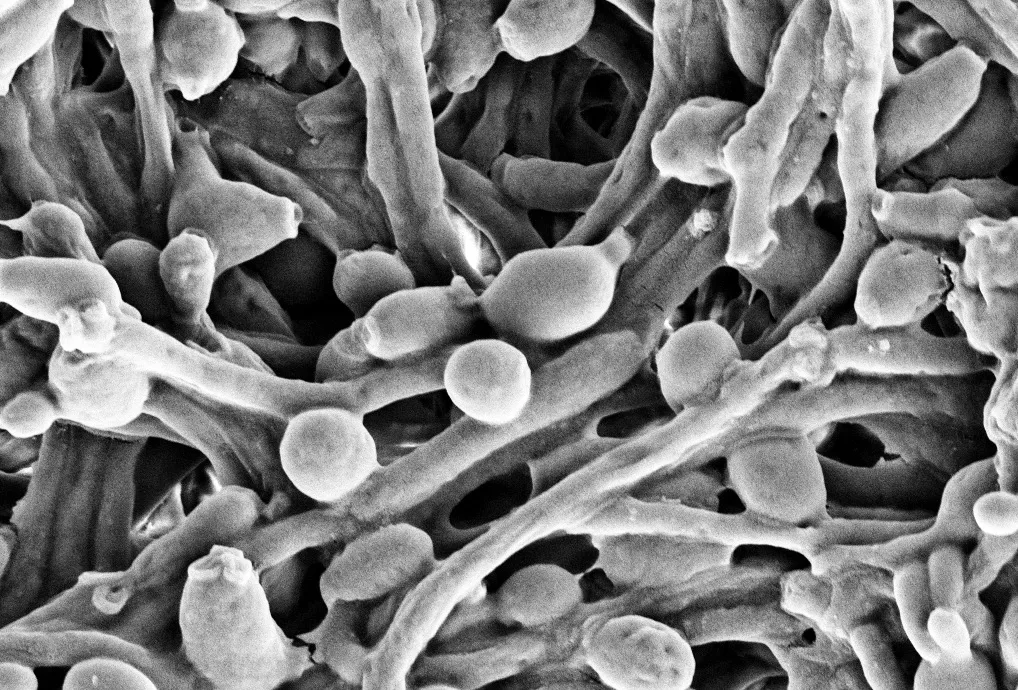
“This study reveals new insights into how host antibodies preferentially allow the non-invasive form of Candida to grow, and could lead to therapies which can help to keep Candida in a commensal (friendly) state,” Dr Elisabeth Lowe from the Biosciences Institute at Newcastle University told BBC Science Focus.
“This is really important for patients who we know are at high risk of invasive Candida infection, such as those on immune-suppressants.”
She added: “We know that in people who get invasive Candida infections, these strains usually escape from the gut. So, helping us to understand how Candida lives and thrives in the gut is essential for developing new treatments.”
This study is part of the growing understanding of how fungi could be used to improve the microbiome of humans – and their overall health.
As Tim Spector, professor at King’s College London and author of Spoon-Fed, told BBC Science Focus: “Fungi are a crucial part of our gut microbiomes that play a key role in our immune defences,but have been hard to measure.
“The next few years should show us how to harness their potential to prevent diseases and how to manipulate them with diet.”
About our experts
Tim Spector is a professor of Genetic Epidemiology at King's College London University. He is also the author of The Diet Myth and Identically Different: Why You Can Change Your Genes.
Dr Elisabeth Lowe is a research fellow at Newcastle University. She studies the relationship between gut bacteria and fungi that colonise the gut. Studies she has contributed to have appeared in journals Nature Microbiology and the Journal of Biological Chemistry.