A new form of sea power is on the horizon after researchers developed a membrane that can harvest energy from seawater.
The technology relies on the process of osmosis, where water or solutes (e.g. salt) move across a semi-permeable membrane, in order to balance the concentration.
There are two main ways in which this process can generate power. If water moves across a membrane, it creates a pressure on one side, which can be converted into energy. If, on the other hand, charged solute particles (‘ions’) move across a membrane, the movement of ions can be harnessed to generate a voltage.
These methods of ‘osmotic power’ – which emit zero emissions, and are less variable than solar and wind energy – could be used in places where freshwater (i.e. a river) meets the sea.
However, despite first being proposed in the 1970s, the technology has so far failed to reach its potential. The main difficulty lies in developing a membrane that’s both efficient and able to last a long time in corrosive seawater.
Now, a team led by scientists at Deakin University in Australia and the University of Michigan in the US have developed a membrane that ticks both boxes.
Read more about the future of energy:
- Marine cultivation technology opening the door to the rich sources of clean energy in our oceans
- Where next for nuclear energy?
- Dr Melanie Windridge: “Fusion energy is cutting edge. It’s exploration. It’s going places people have never been before”
They took inspiration from biology. Cartilage – the elastic tissue that’s found in our bodies – lets ions through with ease. Bone, on the other hand, doesn’t allow ions through, but is strong and stiff.
"We found a way to ‘marry’ these two types of materials to obtain both properties at the same time,” says Prof Nicholas Kotov at Michigan.
The researchers made their membrane using synthetic ‘aramid’ nanofibers, which provide a flexible material similar to cartilage, layered with boron nitride, which is strong like bone.
When the membrane was used to separate two salt solutions of different concentrations, it let through the salt’s positively charged sodium ions but repelled the negatively charged chloride ions, creating an electric current.
The membrane was still functioning after 200 hours, and was able to withstand a wide range of conditions.
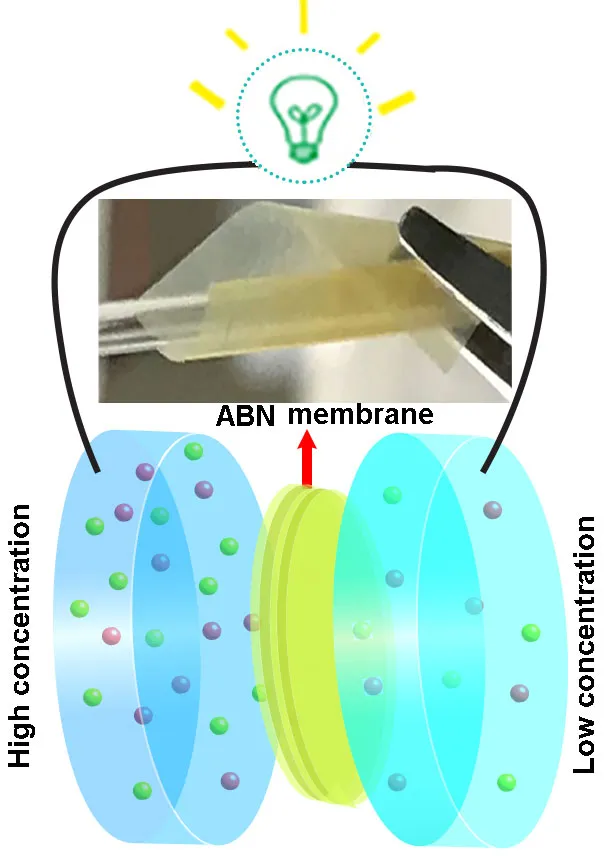
“Our new composite membrane has … high stability at temperatures ranging from 0 to 95 degrees Celsius and at a pH of 2.8 to 10.8,” says Dr Weiwei Lei at Deakin.
“These are the best performing membranes known so far,” adds Kotov.
The researchers believe that their technology is scalable, as both of the main components are inexpensive.