Any coronavirus vaccines made in Europe that are ready for use before the end of the year will make it to the UK before Brexit, the manufacturing lead for the UK’s vaccine taskforce has said. While the UK leaving the EU adds “complexity” to the process, robust plans are in place to mitigate Brexit.
The first doses of the Pfizer/BioNTech vaccine have already arrived from Belgium, and if approved, the initial doses of the Oxford jab are due to be dispatched from Germany to the UK. Later batches of the Oxford coronavirus vaccine will be manufactured in the UK.
“All the vaccines that will be available prior to Christmas, and the end of the year, will get to the UK so that we get them into the country while we’re still in [the EU]," Ian McCubbin, manufacturing lead for the taskforce, said: “In the very very short term we will bring these vaccines into the country before Brexit actually happens.”
Speaking at a press briefing, Steve Bates, chief executive of the BioIndustry Association, added: “I think in terms of Brexit it’s a known risk that we’ve been managing in this programme, along with many, many others.
“Of course, it adds complexity to the process, but there is a robust plan for alternative routes, and mitigation for those, in place. They’ve not been tested yet but we’ve been working on them for some time.”
Read the latest coronavirus news:
- 20-minute COVID-19 test highly effective, trials suggest
- COVID-19 Christmas rules: "Benefits justify the risks", expert claims
The UK has secured more than 350 million doses of seven different vaccines, including 40 million doses of the Pfizer/BioNTech vaccine, and 100 million doses of the jab developed by Oxford and AstraZeneca.
Kate Bingham, chairwoman of the vaccine taskforce was asked if more doses of any vaccines could be bought, if necessary.She replied: “It depends by contract. So some we have options to extend and others we’ve got fixed numbers of doses.”
The members of the taskforce were also asked if committing to so many of the Oxford doses meant the UK had tooled itself up to manufacture the less popular vaccines.
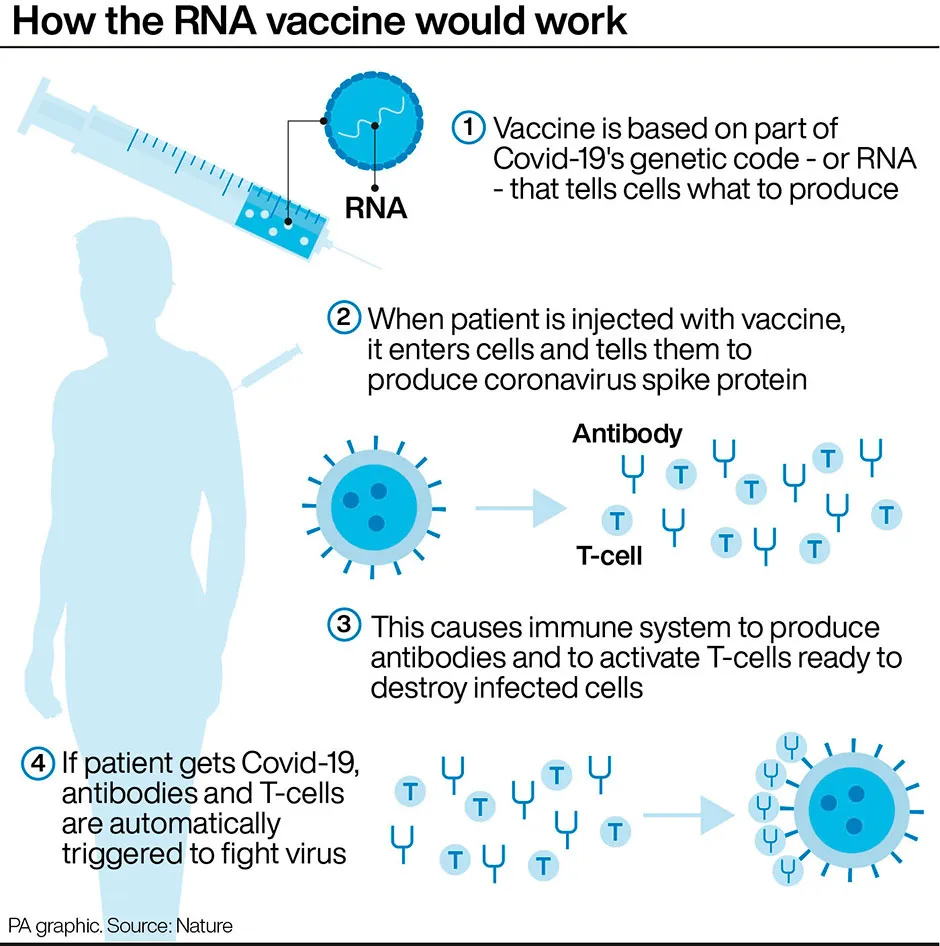
The Moderna and Pfizer vaccines have both shown efficacy of more than 90 per cent and are based on mRNA technology.
Conventional vaccines are produced using weakened forms of the virus, but mRNAs use only the virus’s genetic code.An mRNA vaccine is injected into the body where it enters cells and tells them to create antigens which prepare the immune system to fight coronavirus.
The Oxford vaccine is an adenoviral vaccine, which uses a harmless, weakened version of a common virus which causes a cold in chimpanzees.
While the experts agreed that mRNA vaccines are the most exciting thing that has happened in vaccinology in a long time, they said a lot is still not known about them. While they have been shown to protect against disease, it is not known if they stop infectivity, or transmission, and little is known about their long-term safety.
Bingham said that in the new year trials will begin to look at heterologous boost – whether the combination of two different vaccines can be more effective than just one, or mean the doses can go further.
“We’ll try priming with one vaccine and boosting with another," she said. "And that will be again part of the clinical exploration to see can you broaden and deepen and strengthen the immune response by mixing and matching. And again, that would be potentially with mRNA but it could also be obviously with the other vaccine formats as well.”
How do scientists develop vaccines for new viruses?
Vaccines work by fooling our bodies into thinking that we’ve been infected by a virus. Our body mounts an immune response, and builds a memory of that virus which will enable us to fight it in the future.
Viruses and the immune system interact in complex ways, so there are many different approaches to developing an effective vaccine. The two most common types are inactivated vaccines (which use harmless viruses that have been ‘killed’, but which still activate the immune system), and attenuated vaccines (which use live viruses that have been modified so that they trigger an immune response without causing us harm).
A more recent development is recombinant vaccines, which involve genetically engineering a less harmful virus so that it includes a small part of the target virus. Our body launches an immune response to the carrier virus, but also to the target virus.
Over the past few years, this approach has been used to develop a vaccine (called rVSV-ZEBOV) against the Ebola virus. It consists of a vesicular stomatitis animal virus (which causes flu-like symptoms in humans), engineered to have an outer protein of the Zaire strain of Ebola.
Vaccines go through a huge amount of testing to check that they are safe and effective, whether there are any side effects, and what dosage levels are suitable. It usually takes years before a vaccine is commercially available.
Sometimes this is too long, and the new Ebola vaccine is being administered under ‘compassionate use’ terms: it has yet to complete all its formal testing and paperwork, but has been shown to be safe and effective. Something similar may be possible if one of the many groups around the world working on a vaccine for the new strain of coronavirus (SARS-CoV-2) is successful.
Read more: