The UK government is piloting a coronavirus saliva test that could become an alternative to the existing invasive, and sometimes painful, deep nasal and throat swab.
The new test only requires the individual to spit into a sample pot to be tested for current COVID-19 infection, the Department of Health and Social Care (DHSC) said.
The trial is due to be launched in Southampton this week – and over 14,000 people working in GP surgeries, universities and in other frontline roles have been recruited for the first phase.
Read more about coronavirus testing:
- Coronavirus test: Portable kit could test 16 NHS staff in just 50 minutes
- New coronavirus test 'highly specific and 100 per cent accurate'
- Coronavirus test that uses lasers available 'within a year'
The project is being jointly led by Southampton City Council, the University of Southampton and the NHS, the DHSC said, with the help of other public services in Hampshire.
Participants will receive test results within 48 hours and details of those who test positive shared with the NHS Test and Trace Programme.
There have been fears the existing swab test could be yielding a significant level of false negatives, potentially due to the difficulty in swabbing the sinuses and back of the throat.
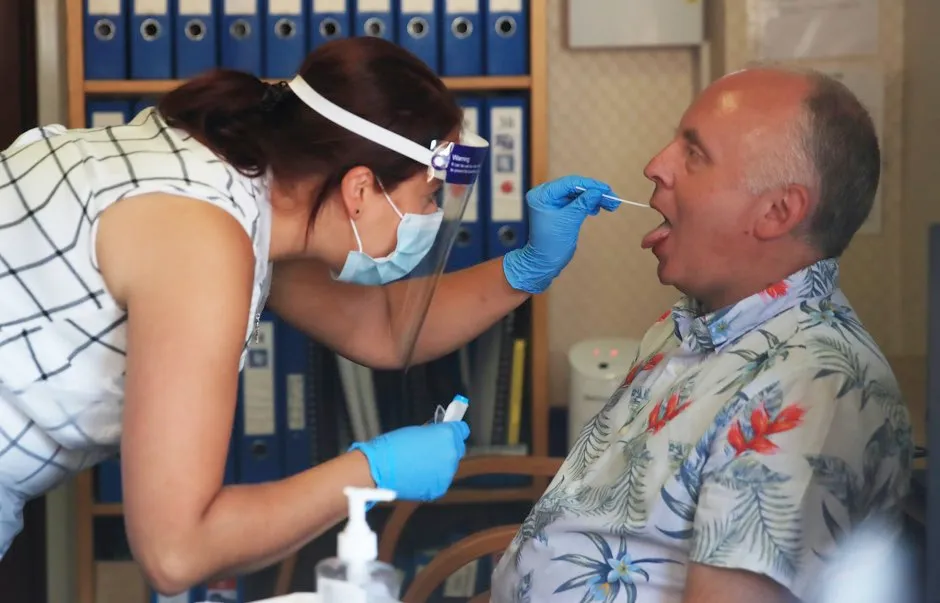
Research from Bristol University and John Hopkins university has found up to 20 per cent of swab tests return false negatives.
It also provokes coughing and spluttering, putting health workers – already working in close quarters with the testee – at even greater risk of the droplet-borne infection.
It is hoped the new tests will significantly boost existing testing capacity and accessibility.
Health Secretary Matt Hancock said: “Saliva testing could potentially make it even easier for people to take coronavirus tests at home, without having to use swabs. This trial will also help us learn if routine, at-home testing could pick up cases of the virus earlier.”
He added: “I am very grateful to everyone involved in the trial who is helping us develop our understanding of the virus which will benefit not only our but the global response to it.”
Your coronavirus questions answered:
- How can I protect myself from the coronavirus when shopping?
- Can I get the coronavirus from a parcel?
- Can I get the coronavirus twice?
The test was developed by biomedical firm Optigene, and the DHSC said it is also looking at other non-swab, saliva-based tests from four other companies.
It said it is also working with a number of manufacturers ready to scale up production of the kits.
Professor Keith Godfrey, from the University of Southampton, said: “The health, social and economic impacts of lockdown cannot be underestimated.
“Through this initiative we believe we can contribute to safely restoring economic activity within the city and region during national relaxation measures, whilst enabling people to regain their lives, work and education.”
The pilot is due to run for four weeks with testing on a weekly basis.
How do scientists develop vaccines for new viruses?
Vaccines work by fooling our bodies into thinking that we’ve been infected by a virus. Our body mounts an immune response, and builds a memory of that virus which will enable us to fight it in the future.
Viruses and the immune system interact in complex ways, so there are many different approaches to developing an effective vaccine. The two most common types are inactivated vaccines (which use harmless viruses that have been ‘killed’, but which still activate the immune system), and attenuated vaccines (which use live viruses that have been modified so that they trigger an immune response without causing us harm).
A more recent development is recombinant vaccines, which involve genetically engineering a less harmful virus so that it includes a small part of the target virus. Our body launches an immune response to the carrier virus, but also to the target virus.
Over the past few years, this approach has been used to develop a vaccine (called rVSV-ZEBOV) against the Ebola virus. It consists of a vesicular stomatitis animal virus (which causes flu-like symptoms in humans), engineered to have an outer protein of the Zaire strain of Ebola.
Vaccines go through a huge amount of testing to check that they are safe and effective, whether there are any side effects, and what dosage levels are suitable. It usually takes years before a vaccine is commercially available.
Sometimes this is too long, and the new Ebola vaccine is being administered under ‘compassionate use’ terms: it has yet to complete all its formal testing and paperwork, but has been shown to be safe and effective. Something similar may be possible if one of the many groups around the world working on a vaccine for the new strain of coronavirus (SARS-CoV-2) is successful.
Read more:

