As plenty of scuba divers will tell you, they rarely find themselves in exactly the right place at the right time to spot a whale shark, a basking shark, a hammerhead or any other shark species gracefully gliding by. Shark researchers face this same dilemma in their studies. They’ll spend days and weeks at sea hoping for shark encounters. Conventional surveys involve scuba diving, setting fishing lines to catch sharks or lowering video cameras into the sea, along with chunks of bait that sharks might come up and chew. But now there’s a much quicker and cheaper shark tracking tool – a bucket-full of seawater.
“The ocean is a soup of DNA,” says Judith Bakker, a postdoctoral researcher at Florida International University. “Everything from small plankton to gigantic whales are constantly leaving behind traces of DNA.”
Read more 2019 science breakthroughs:
Small snippets of genetic material come from mucous, skin cells, urine and faeces. Over the last 10 years, scientists have worked out how to gather these small DNA fragments – known as environmental DNA or eDNA – and convert them into a powerful tool to track species without actually seeing them.
In a study spanning the Pacific Island of New Caledonia and various spots in the Caribbean, Bakker screened water samples for shark eDNA. “A couple of years ago, people were very sceptical,” she says. The eDNA technique was originally developed to study soil microbes and later in streams and ponds, searching for invasive species like American bullfrogs. It took a while to catch on with marine scientists, as they assumed there wouldn’t be enough DNA to detect species swimming through these vast bodies of water. But Bakker and her colleagues took on the challenge and eventually fine-tuned the technique for detecting sharks. “Many shark populations are overfished, they’re threatened,” says Bakker. The first step for effective conservation programmes, she explains, is knowing which species are present and eDNA could help do just that.
First get the DNA
The technique Bakker uses is essentially the same for all aquatic eDNA studies. First, she motors out to each sampling site at sea, scoops up a few litres of water and pours it through a fine filter to snag the DNA fragments from all the species that have recently swum by. Back in the lab, Bakker adds a primer that picks out of the mix all the fragments of shark DNA. Primers are short DNA or RNA strands that act like highly specific strips of Velcro and stick only to particular DNA sequences, in this case only the codes specific to sharks. Next, she makes multiple copies of the shark eDNA fragments and feeds them into a high throughput sequencing machine. After 24 hours or so the machine produces huge data files with masses of DNA sequences, which she then compares with known DNA databases to work out which species the eDNA fragments came from.
Her studies have so far detected 21 shark species and shown that parts of the oceans protected from fishing have a greater shark diversity. In the Bahamas, where commercial shark fishing is banned, Bakker found eDNA from 11 species. In less well protected waters of Jamaica and Belize, she only found a couple of different sharks.
A major benefit of eDNA is that it’s not invasive – you don’t have to catch and handle sharks, which can stress them out. Also, the technique avoids any bias caused by behavioural quirks of different species. “If you go fishing, you may catch five tiger sharks,” says Bakker, “but perhaps you don’t catch the bull sharks because they’re just not attracted to your fishing line.”
Another recent study in New Caledonia, led by Germain Boussarie from the University of Montpellier, showed just how well eDNA outperforms other surveys. Water samples collected during two weeks of fieldwork provided DNA that identified more shark species than were detected in two years’ worth of underwater baited camera studies and thousands of scuba dives. The eDNA samples found sharks 90 per cent of the time, compared to only 50 per cent in camera studies, and 15 per cent in dive surveys.
But still some sharks are missing. Working in the Caribbean, Bakker and her colleagues regularly saw nurse sharks but failed to find any traces of their eDNA. The problem comes down to the primers. “They just don’t like to attach to nurse shark DNA,” says Bakker. Fixing this primer mismatching is one of the improvements she hopes to see in eDNA techniques in the coming years.
There’s a goldilocks quality to DNA fragments in water: it doesn’t vanish too quickly or linger too long to be useful. If the eDNA of a tiger shark is found, then one probably swam nearby in the last day or two. Any longer and DNA gets broken down by saltwater, UV light and bacteria until the fragments are too small to detect. Bakker is hopeful that advances in the technique will soon allow her not only to work out which sharks are there, but also how many of them. For now, eDNA can only give a rough guess at abundance. “We can say that most likely there’s a lot more sharks in this area than that area,” she says.
Off the coast of Greenland, a study has shown that eDNA could revolutionise the way fisheries are monitored. Researchers collected water samples and extracted eDNA from more than 30 fish families, including many that are important in commercial fisheries. The team found that the abundance of eDNA broadly matched the amount of fish they caught in conventional trawl surveys in the same area. The new technique could also help survey species that rarely show up in trawl nets. They found lots of eDNA from Greenland sharks, illusive giants that can live for 500 years. But the trawl surveys only caught one Greenland shark, suggesting that many others avoid the nets.
Call in the drones
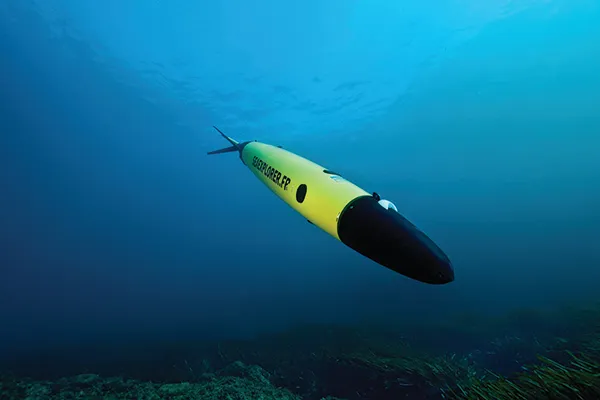
In the next few years, fisheries scientists could start to abandon trawl surveys and instead use eDNA to count fish. And to collect water samples, scientists might even throw away their buckets and start sending out fleets of drones and underwater robots to get water for them. Already there are ocean gliders that look like a cross between miniature submarines and aeroplanes, fitted out with scientific sensors and instruments. They can be programmed to navigate on their own for thousands of kilometres through the seas, gathering measurements as they go, like salinity and temperature. Equipped with water sampling devices, these robots could become the research tool of choice for eDNA studies, opening up remote stretches of the oceans that are otherwise difficult and expensive for scientists to get to, including down in the deep sea, many kilometres beneath the waves.
Alien species surveillance

Environmental DNA makes an ideal early warning system for alien invasive species – creatures that find themselves in places they aren’t expected or wanted. Given half a chance, many successful invaders will rapidly multiply and unleash economic and environmental havoc, like zebra mussels that block intake pipes of water treatment plants and Chinese mitten crabs that crumble riverbanks with their burrows. DNA fragments can show when a potentially invasive species is present in small numbers and hasn’t yet become a pest. The sooner an invasion is discovered, the more likely control measures are to work.
From pondweed and clams, to fish and sponges, eDNA is used to monitor all sorts of invasive species. In the US, eDNA helps conservationists quickly find out if an invasive European pig has wallowed in a pond, or if it remains pig-free. With climate change, invasions are likely to become more problematic as temperatures rise and more species try to compensate by shifting their ranges.
We’re already seeing the spread of species into British waters, such as the carpet sea-squirt, a gloopy, fast-growing animal that smothers other sealife, including oysters and mussels in shellfish farms.
Hunting for ancient beasts
For land-based scientists, a handful of soil can tell them which animals have crawled through it or flapped their wings overhead. A chunk of permafrost can even reveal whether, thousands of years ago, a woolly mammoth trudged past. A study of eDNA in Alaska suggests that mammoths were still alive until 10,500 years ago, almost 2,000 years later than previous estimates based on mammoth bones. If this new extinction date is accurate, it means humans and mammoths co-existed for several millennia.
In the Bahamas, where commercial shark fishing is banned, Bakker found eDNA from 11 species
Insights into past climates and ecosystems also come from eDNA. Ice cores taken from Greenland contain traces of eDNA from conifer trees and insects, showing that the land now lying under more than two kilometres of ice sheet, was once a flourishing forest.
This cutting-edge technology could also finally solve a centuries-old puzzle. An international group of scientists, calling themselves the Super Natural History team, is probing Loch Ness in Scotland. They plan to find out once and for all if a monster really does lurk in the dark depths. The eDNA sequences they decode should show if any unknown species are living down there, while also revealing a bigger picture of the biodiversity in this, the UK’s biggest body of freshwater.

Follow Science Focus onTwitter,Facebook, Instagramand Flipboard