“It’s like a crawly feeling inside,” says Judy*. “You get hot, then chilled, and you feel like you want to run away.” The 57-year-old has short dark-grey hair and a haunted expression. She’s breathless and sits with her right leg balanced up on her walking stick, rocking it back and forth as she speaks.
Judy explains that she suffers from constant, debilitating pain: arthritis, back problems, fibromyalgia and daily migraines. She was a manager at a major electronics company until 2008, but can no longer work. She often hurts too much even to make it out of bed.
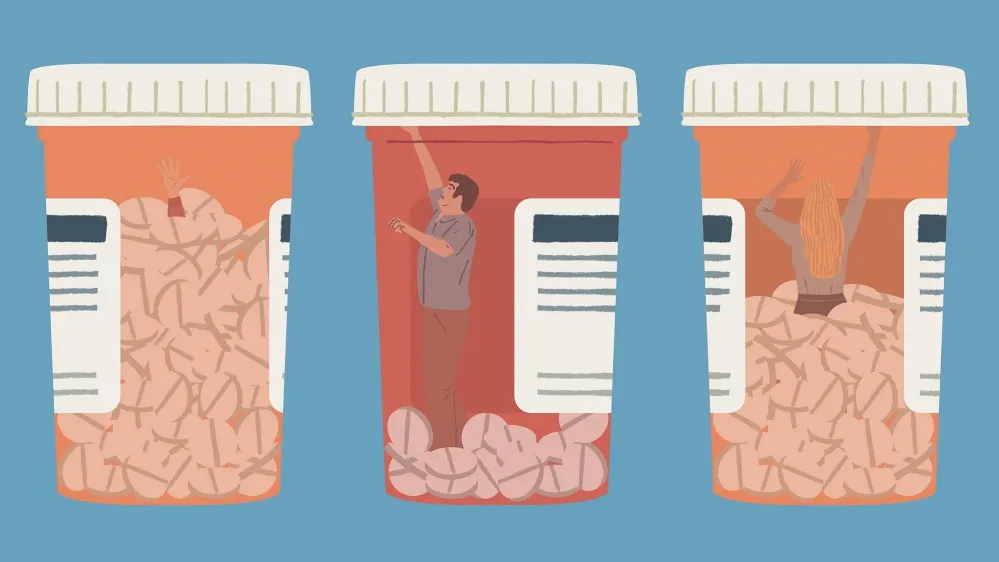
She’s taking around 20 different medications each day, including painkillers, antidepressants, sedatives and a skin patch containing a high dose of the opioid drug fentanyl, which she says did not significantly help her pain and which she’s now trying to come off. Her physician has been tapering the dose for months, so in addition to her pain she suffers withdrawal symptoms: the chills and crawling dread. Then her clinic announced that it would no longer prescribe any opioids at all, the unintended result of new, stricter measures aimed at clamping down on opioid abuse. Faced with losing access to the drug on which she is physically dependent, she has come to another clinic, Pain Consultants of East Tennessee (PCET) in Knoxville, desperate for help.
Ted Jones, the attending clinician, calls patients like Judy “refugees”. He says that he sees “tons” of similar cases. Over 100 million Americans suffer long-term pain. Now they find themselves at the epicentre of two colliding health catastrophes in the USA: chronic pain and opioid abuse.
Over the last few decades, US doctors have tackled constant pain problems by prescribing ever-higher levels of opioid painkillers – drugs such as hydrocodone and oxycodone, which belong to the same chemical family as morphine and heroin. These medications have turned out to be less effective for treating chronic pain than thought – and far more addictive. The surge in prescriptions has fed spiralling levels of opioid abuse and tens of thousands of overdose deaths.
Efforts to curb opioid prescriptions and abuse are starting to work. But with the spectacular failure of a drug-centric approach to treating chronic pain, doctors desperately need alternative ways to fight a condition that blights millions of lives. Jones is trying one, seemingly unlikely technological solution: virtual reality.
§
Since the 1990s, opioid prescriptions have tripled in the USA, which has less than 5 per cent of the world’s population but now uses more than 80 per cent of the world’s opioid supply – with devastating consequences.
Opioid painkillers were previously used only in specialist cases, such as short-term pain after surgery or terminal cancer pain. But a campaign by the American Pain Society arguing for pain to be treated more aggressively – as well as marketing from pharmaceutical companies, claiming that newly approved opioid drugs such as OxyContin were effective and non-addictive – resulted in doctors prescribing them much more widely for many different types of chronic pain. “We were told pain was undertreated,” says Joe Browder, a physician and senior partner of PCET. “There was no upper limit.”
The new drugs turned out to be highly addictive. In 2015,more than 16,000 peopledied from overdoses of prescription painkillers, in what figures from the US Centers for Disease Control and Prevention (CDC) show is the USA’s worst ever drug epidemic.
Tennessee is one of the worst-hit states in terms of opiate prescriptions and overdose deaths. Part of the state lies in the Appalachian mountains – a rural region that has long struggled with economic deprivation and lack of jobs, caught in what Karen Pershing of the Metro Drug Coalition in Knoxville describes as a “culture of addiction and poverty”. The narcotic pills prescribed by doctors have flowed easily from pain clinics into the surrounding community. “People couldn’t afford street drugs,” says Pershing. Suddenly they had access to prescription medications that provided the same psychological escape.
In particular, there was an explosion of unregulated but legal pain clinics known as ‘pill mills’. All that was needed was for the director of a clinic to be a qualified medical doctor. Staff could then hand out prescriptions for opioid painkillers in return for cash. “Every time you turned around there was another one,” says Pershing. Some of them were in old houses, with handwritten signs. People would be in and out in a few minutes, selling on the phone as soon as they got out.
Over the last five years or so, there have been a series of measures aimed at curbing opioid prescriptions and abuse, from requiring pain clinics to be run by doctors certified in pain treatment, to encouraging the public to report suspicious activity by text message. In some states, including Tennessee, physicians must regularly count patients’ pills to ensure they’re not taking too many or selling them on, and face criminal charges if patients abuse their drugs. Not surprisingly, some clinics now refuse to prescribe opioids at all.
PCET is in one of the hardest-hit areas. According to theUS Senate Committee on Health, Education, Labor, and Pensions, Tennessee ranks third in the nation for prescription drug abuse, with 5 per cent of the population abusing these drugs. Here in Knox County, there were 133 deaths from opioid overdoses in 2014, a higher death rate than any other county in the state.
Physicians at PCET do still use opioid drugs, though with strict precautions. For example, Jones runs courses to help patients treat their pills responsibly and stay within the law. “Take $1,000 cash and put it with your pain meds,” he tells them. “Or put a loaded gun there. If cash or a gun isn’t safe there, your pain meds aren’t safe there.” (Rule of thumb: “If they leave you some, it was family. If they clean you out, it wasn’t.”)

He also interviews new patients like Judy to assess how likely they are to abuse their medication. Other refugees chasing opioids this morning include Scott*, a balding teetotaller in his 60s who runs a barbecue joint. Injuries from working at rodeos and horse shows in his youth as well as nerve damage from recent chemotherapy mean “if I’m moving, I’m hurting”. Meanwhile Debra*, a 65-year-old widow with turquoise shorts, suffers from lupus, arthritis, fibromyalgia, diabetic neuropathy and sciatica, not to mention anxiety and depression. “I hurt everywhere,” she says. She hides her drugs around the house but says her drug-addict daughter still finds them. Jones puts her at medium risk for misusing. She’ll still get opioids, but will have a drug screen every month or two to make sure she’s not taking too much.
Measures like these are starting to work. Between 2012 and 2015, there was a12–18 per cent dropin the amount of opiate painkillers prescribed nationally. But that leaves many patients struggling to cope without these drugs, while more Americans than ever – some estimates say over 100 million – are in chronic pain, costing the US economy around $600 billion every year. (Although other countries haven’t seen such a large spike in opioid use as the USA, chronic pain is a huge and growing problem globally, affecting 15–20 per cent of adults in industrialised nations.)
Despite taking their drugs responsibly, the patients Jones sees today are all still desperate with pain. Listening to their stories, the overwhelming impression is of chronic pain not as a discrete physical condition that can be solved with a pill, but as a diffuse suffering that grows and spreads around the body, evading the efforts of ever-increasing medications and often entwined with arduous life histories and psychiatric issues such as anxiety and depression. “Pain and anxiety are intertwined,” confirms Jones. “It’s hard to know when you are treating anxiety or pain.” Physicians often ask patients to rate their pain out of ten, but “the pain score is really a distress score,” he reckons. “It’s always a seven. It’s a measure of depression, unhappiness and pessimism.”
Browder says he realised more than a decade ago that ever-higher drug doses weren’t the answer. Patients may not have been reporting as much pain, but the drugs weren’t doing anything, he says. Instead of trying to eliminate pain with narcotics and sedatives, Browder and his colleagues decided to prioritise patient function. “It’s all about getting people to do more in their life with the pain they have,” explains PCET medical director James Choo. “How do you get people to do more, to live more, to be more like the people they were before they started hurting?”
They started to reduce the amount of opioids they prescribed, emphasising other medical interventions such as steroid injections, nerve ablation and spinal cord stimulators. The clinic also started offering physical therapy and occupational therapy, while an embedded team of psychologists, including Jones, offers counselling as well as advice in proven pain-relief techniques such as cognitive behavioural therapy, distraction and relaxation mindfulness.
Persuading patients to embrace this more diverse approach isn’t easy, however. Relaxation techniques work well, but they take practice, and Jones says they’ve struggled to attract patients to multi-session courses on techniques for coping with pain. A few people will do it, but in general life gets in the way, he says, adding that there are a million reasons why they don’t come. People don’t want programmes, agrees Choo. “They just want to take a pill.”
§
One day last winter, Jones came to work to find an empty clinic; heavy snowfall had kept many patients at home. He filled time by surfing the internet, and stumbled across the website of a technology start-up called Firsthand Technology, based 2,000 miles from Knoxville in San Francisco.
For the company’s CEO, Howard Rose, virtual reality – or VR – is nothing less than a superpower, or as the company website puts it, a “high-bandwidth channel” into our brains that can transform how we see ourselves and the world. Within a couple of generations, he predicts, VR will be woven into every aspect of our lives. He’s starting with how we manage pain.
Rose began working in VR more than 20 years ago, at the Human Interface Technology Lab (HITLab) at the University of Washington in Seattle. The lab was founded by Tom Furness, who had pioneered the use of VR when working for the US Air Force in the 1960s. Under Furness, Rose and his colleagues developed civilian uses of the technology, creating VR worlds for everything from treating spider phobias to teaching Japanese. One of the most successful products to come out of the lab was SnowWorld, developed by cognitive psychologist Hunter Hoffman to ease the pain of patients with severe burns.
Burns patients have to undergo regular wound-care sessions so painful that they can be excruciating even with high doses of painkillers. SnowWorld was designed as a kind of souped-up distraction method for use during these sessions, to divert patients’ attention away from their pain. Adapted from flight simulation software, it creates the experience of flying through a virtual ice canyon while exchanging snowballs with penguins and snowmen.
Over the past ten years or so Hoffman and his colleagues have shown in several trials, including onarmy veteransburned by explosive devices in Iraq and Afghanistan, that this works. Playing SnowWorld during wound-care sessions eases patients’ reported pain up to 50 per cent in addition to the pain relief they get from drugs – significantly better than other forms of distraction, such as music or video games. Studies also show that SnowWorld reduces activity in areas of the brain associated with pain perception.
The researchers believe that the sense of immersion created by VR – feeling physically present in the virtual location – is crucial. “It works because it tricks your senses into perceiving that the computer-generated environment is real,” says Rose. “VR becomes a place you are, not something that you are watching.”
Thanks to Hoffman’s research, SnowWorld has become famous, featuring in numerous press reports and even in anoff-Broadway play starring Hollywood actress Mamie Gummerthat isnow showing at the National Theatre in London. Research interest has grown too, and VR immersion has since been shown to reduce reported pain and distress during a range of medical and dental procedures, from chemotherapy to taking blood.
Meanwhile, Rose and his colleague Ari Hollander left academia to form Firsthand Technology. SnowWorld is a research tool; they wanted to develop commercial products. They built Cool!, which Rose describes as “a sort of next generation of what we learned in SnowWorld”. Featuring more interactivity and a wider variety of environments, it’s more open-ended, he says, “a kind of playground”.
I tried it myself at the PCET clinic. A few seconds after slipping on the headset, I was floating along a river with grassy banks. There were mountains in the distance, and a blue sky with scattered clouds. Along the edge of the water, fluffy brown otters stood greeting me on their hind legs. Using two hand controllers, I threw fish to them and they rolled over in delight, changing colour to zebra stripes or flamingo pink. Then I launched huge bubbles that bounced pleasingly around the landscape.

The graphics were detailed, if a little cartoonish, but what struck me most was the physicality of the experience – to my brain, this otter-inhabited world wasn’t simply something I was watching, but a place that I was actuallyin. When I passed under a rocky bridge, I flinched. When snow fell, I felt the exhilaration of clear, cold air.
VR is undoubtedly effective at shifting attention, but Rose argues that it works on other levels too. “We know that if people feel anxious and helpless then their suffering from the pain is much greater,” he says. Mentally taking people to a distant, safe place reduces their anxiety, he says, while interactivity – the ability to move around an environment and throw snowballs, for example – helps them to feel more in control.
He’d wondered whether these attributes might help patients with chronic conditions too – those suffering from pain, anxiety and helplessness in daily life. Then he received an email from Jones in Tennessee. “We’ve got patients, you’ve got a product,” said Jones. A few months later, Rose flew to Knoxville and dropped off his equipment. But would it work?
§
Christine* smiles. “Mmmm,” she says. “You can almost feel the petals hitting you.” The 69-year-old is sitting in Jones’s green leather armchair, her eyes hidden by a bulky black visor.
Jones’s laptop shows Christine’s field of view as she floats down the stream. She looks around from the otters to some mysterious balls of dancing coloured flame, then scrunches up her face as she brushes past the blossom-laden branches of a grove of cherry trees.
Christine has led an active life: she used to run a tour company in Mexico, before becoming a chef at her local synagogue. But despite her “very full” life – which has included regular childcare – she now finds it difficult to focus on anything but her pain. Since 2007 she has suffered from an autoimmune condition that attacks her nerves, causing burning pain down both legs and across the soles of her feet. She is also recovering from back surgery. She has resisted opioids and stopped taking two other drugs, Lyrica and Cymbalta, because they interrupted her thinking and speech. “It spoiled my life,” she says. Now she gets by on Advil (ibuprofen) and lidocaine patches, which she wears on her legs during the day and on her feet at night, but the pain never goes away. “It’s all you think about,” she says.
When she arrives in Jones’s office, Christine rates her pain as 7/10. Then she puts on the headset and headphones for her third session of VR. “I’m already relaxing,” she says. After the cherry trees she follows the river into a cave, its rocky walls covered with strange drawings and sparkling gems. How’s your pain, I ask. “Oh,” she says, as if surprised to be reminded of it. “Zero. It’s gone.”
Compared to other forms of distraction, such as colouring or watching TV, VR “works a whole lot better,” Jones says. “It grabs your attention. You just put the helmet on and you are gone.”

Jones has recently completed two small clinical trials of Cool!, which together involved 40 participants receiving between them around 60 sessions of VR. Only one person didn’t report reduced pain, he says. Overall, the patients reported that their pain fell by 60–75 per cent (compared to baseline) during their VR session, and by 30–50 per cent immediately afterwards. The best morphine does is 30 per cent.
It’s early research, but a few other studies of chronic pain have found similar results. Earlier this year, Diane Gromala of the Pain Studies Lab at Simon Fraser University, Canada, and her colleagues reported on a VR game called Cryoslide – inspired by SnowWorld – which involves sliding through a snowy landscape and icy cave while throwing snowballs at fantasy creatures. When chronic pain patients played it for ten minutes, their pain reduced significantly compared to those asked to use other distraction strategies such as meditating, reading or playing games on a phone. Elsewhere, Brenda Wiederhold and colleagues at the Virtual Reality Medical Center in San Diego found that chronic pain patients immersed in virtual scenes such as forests, beaches and mountains reported significantly reduced pain compared to baseline. They also had a reduced heart rate and raised skin temperature, suggesting that they were more relaxed. According to the published paper, patients said things like: “This is the first pain relief I have had in three years” and “I was so busy playing the game, I forgot about my pain”.
No peer-reviewed studies have yet investigated whether VR is helpful for managing chronic pain long-term. But the research so far provides the first proof of principle that VR can ease pain in chronic conditions. It isn’t possible or desirable to immerse patients all the time, of course, but even short sessions might provide welcome respite for patients who are otherwise always in pain. Jones says that compared to techniques such as mindfulness, which require long-term effort and training, “with VR you put a visor on their head and they’re there”.
He hopes that the effects last at least a little longer than the session. In his trials, patients reported that their pain relief lasted for between 2 and 48 hours afterwards. This supports the idea that it might be triggering the release of pain-relieving endorphins, for example, which ease symptoms even after the headset is removed. Perhaps it also demonstrates to patients that their pain “is not intractable, it can be influenced,” suggests Browder. In other words, it shows people with grinding, constant pain that there is hope.
That interpretation chimes with the accounts of Jones’s patients. Cindy* is 54 years old and suffers from non-Hodgkin’s lymphoma as well as familial pancreatitis, a condition that killed her brother and sister and which causes severe stomach pains and nerve damage. She’s on 17 medications three times a day. After the VR session, she’s visibly calmer and a noticeable hand tremor has temporarily eased. The VR “is an escape from pain reality,” she says. Her husband agrees: “After she does the VR, her mood lightens,” he says. “She’s more upbeat towards the world.”
Andrew*, a tattoo-covered ex-Green Beret, suffers pain as a result of nerve damage from exposure to chemical weapons in Iraq, combined with back problems “from jumping out of planes and carrying a heavy rucksack”. Opioid painkillers haven’t helped and he has often contemplated suicide, but the VR takes it all away, he says. “It made me feel the way a man feels after achieving an orgasm.”
§
There is increasing interest in using VR to teach long-term coping skills that patients can use in their daily lives, or as Rose puts it, “to change that relationship with the pain”. One way to do this is to combine the VR with biofeedback, creating environments that change according to a person’s physiological state. For example, Gromala and her colleagues have developed a virtual environment aimed at teaching mindfulness-based stress reduction (MBSR). There is robust evidence that MBSR reduces chronic pain, but it takes time and effort to learn. Gromala’s “virtual meditative walk” takes users along forest trails as they listen to a guided meditation. Biosensors track their arousal level, and modify the weather in response: light fog recedes as the patient relaxes, or thickens if they become more stressed.
In one trial, patients who tried the virtual walk had significantly reduced pain compared to those who listened only to the audio track. Gromala and her colleagues now need to test whether regular sessions enable users to better control stress and pain in everyday life. They don’t just want to distract users from their pain, theywrite in a paper, but to “arm them with a learned capacity to modulate it”.
Meanwhile, Jones is discussing with Rose the idea of a “pain tamer”, in which by reducing their stress levels, patients can make a virtual monster – representing their pain – shrink and disappear. “They learn to relax themselves,” says Jones. “It’s not so much distraction as learning how to calm yourself in the face of an angry adversary.” Rose and Gromala envisage such virtual worlds running on a variety of platforms – where patients might experience high-quality immersive VR in a clinic, supplemented at home by daily top-up sessions on tablets or phones.
“People talk about waves of VR,” says Rose. “They were always little ripples. This is a tsunami.” When SnowWorld was first developed in 1990s, Hoffman and his colleagues used a supercomputer and heavy helmet that together cost $90,000. Today, users can buylightweight high-resolution headsetssuch as the Oculus Rift, which is the size of a scuba mask, for around $600. HTC and Sony offer similarly priced devices, while vastly cheaper sets by Google and Samsung don’t have such impressive displays but can run off tablets and smartphones slotted into cardboard or plastic headgear. For Rose, all this amounts to nothing less than a revolution in medicine.
“The treatment model of medicine is you come in and stuff is done to you,” he says. VR, on the other hand, shifts responsibility and control to the patient. By exploring virtual worlds, those crippled by anxiety or pain may gain the skills, strength and courage they need to transform their experience of the real one.
There are several obstacles to its use becoming routine. One is a reluctance among many hospitals and clinics to adopt unfamiliar technology. Even where the research evidence is strong – in burns patients, for example – VR pain relief is still not widely used. Jones describes how PCET raised funds for a nearby children’s hospital, and demonstrated to one of the nurses how VR could help children going through medical procedures. She wasn’t impressed. “It’s frustrating,” says Jones. “We gave her $15,000 and she’s going to buy more colouring books.”
Developing VR into a routine treatment is also going to require new models of funding. So far, Jones has paid for his clinical trials himself. “It’s my hobby,” he says. “I don’t have a boat.” Beyond those, he actively discourages his physician colleagues from referring patients for VR, despite the fact that he is convinced it would help, because he can’t bill for it. VR pain relief needs a “champion” he says, who will fund trials and convince insurance companies to take it seriously. “The VR industry has got some work to do.”
Meanwhile, he’d just taken delivery of Firsthand Technology’s latest virtual world, called Glow.I tried it, and found myself standing in a moonlit forest clearing in front of what looked like a carved stone altar. Fireflies danced through the air. At Jones’s instruction I held out my hands and focused on breathing calmly, slowly. A sensor on my fingertip relayed my heart rate to the computer so that as I relaxed, hundreds of fireflies settled between my hands, eventually creating a glowing sphere.
Instead of an experience that you play with, says Rose, “this is having the world react to you.” One application is customising an experience to someone’s changing pain. For example, for women in labour, Rose suggests that a virtual world might offer “superpowers and catharsis and blowing stuff up” during contractions, while promoting relaxation in between.
For Jones, there won’t ever be one answer to chronic pain. But VR could be another tool. As well as providing a non-pharmacological alternative to treating pain, it might help people who are on opioids to minimise their dose, giving them something active they can do instead of popping extra pills. “With opioids, you just take it, sit and wait.” That’s why people end up taking more pills than they need – they just want to do something to help their pain.”
*Patients’real names have been anonymised in this article.
Thisarticlefirst appeared onMosaicand is republished here under a Creative Commons licence.
Follow Science Focus onTwitter,Facebook, Instagramand Flipboard