Early on the morning of 30 December 2007, a drunk 41-year-old man left a party in the city of Stokmarknes, in northern Norway. Soon after, he slipped and fell into a steep-sided ditch. Plunged neck-deep in the freezing water, he was unable to get out and spent nearly an hour in the ditch before passers-by spotted him and hauled him out.
Despite their best efforts to warm him up, the man remained severely chilled, largely thanks to the air temperature being a biting -2°C. Shortly after the paramedics arrived, he fell unconscious, stopped breathing and went into cardiac arrest. It would be seven hours before his heart started beating properly again, and for five of those he was technically dead. Somehow the extreme cold – the very thing that had caused the man’s heart attack in the first place – had saved his life.
Read more:
- You think you're cold? Here are some amazing photos of chilly people in freezing places
- What are the 10 coldest places on Earth?
In healthy humans, the body maintains a core temperature of between 36.5 and 37.5°C – anything below this is a dangerous condition known as hypothermia. When someone’s body enters a hypothermic state, their metabolism slows down, their heart rate slows, organs start to shut down and eventually, their heart stops beating. Within a few minutes of the heart stopping, the body’s oxygen reserves are depleted and cells start to produce toxic chemicals. This quickly starts to cause irreversible damage to the delicate tissues of the brain. Even if resuscitation is successful, the danger is not over: most cardiac arrest patients whose hearts are restarted end up dying in hospital from the damage caused by the return of oxygenated blood throughout the body; up to 30 per cent suffer permanent brain damage.
However, there’s an old saying in the medical profession: “No one’s dead until they’re warm and dead”. In cases of cardiac arrest caused by extreme cold, an extraordinary thing can happen: the reduction in body temperature reduces the brain’s need for oxygen. If cooling is rapid enough, it can help prevent toxic chemicals accumulating while the heart has stopped, and continues to protect the brain when oxygenated blood returns.
The Norwegian man arrived at a nearby hospital at 5am, with a temperature of just 25.5°C – easily in the most severe category of hypothermia. After attempts to warm him failed, medics called for a helicopter from the University Hospital of North Norway (UNN), a better-equipped medical centre over 250km away. Doctors continued to do CPR on the man, but by the time the helicopter reached UNN with the patient onboard it was nearly 9am. He had been technically dead for nearly five hours.
At 11.37am, after hours of work by teams of UNN staff, the man plucked from the icy ditch was finally revived, and weaned off machines that had been artificially pumping blood around his body. It had been nearly seven hours since he had entered cardiac arrest – one of the longest resuscitation periods ever recorded. Miraculously, he went on to make a full recovery, with no signs of brain damage at all.
“His metabolism had decreased by 60 to 70 per cent,” says Lars Bjertnaes, a professor of critical care at The Arctic University of Norway, who examined the case in detail. “His oxygen needs probably could be met by a cardiac output of about a quarter of normal.”
Back to the cold school
Stories like this have inspired a range of medical treatments that deliberately induce cold states in patients. It’s seen as a cutting-edge treatment, but reports of doctors using extreme cold to keep people alive actually go back centuries. A paper on ‘the Russian method of resuscitation’ from 1803 describes covering cardiac arrest patients with snow to boost their chance of survival. Meanwhile, in 400 BC, Greek physician Hippocrates advocated packing wounded soldiers in ice and snow when moving them.
Since the 1990s, putting patients into a state of hypothermia has been standard practice in open heart surgery, and in the treatment of babies born with heart defects. Here, doctors must ‘turn off’ the circulatory system in order to operate on the heart; reducing body temperature allows them to do this for long periods without causing tissue damage.
Over the past decade, the use of ‘therapeutic hypothermia’ – also known as targeted temperature management (TTM) – has become widespread in the treatment of heart attacks and strokes. The concept is the same: use low temperatures to protect against the damaging cellular reactions that occur when the oxygen supply is cut off, and perhaps more importantly, prevent damage when blood and oxygen return after treatment.

“We try to reach hypothermia as soon as possible,” says Gladys Janssens, a cardiology researcher at VU University Amsterdam who has studied different methods of cooling heart attack patients. “After reaching hypothermia, we try to keep the temperature as close to the goal temperature as possible for 12 to 24 hours. Lower temperatures can be a risk for bleeding complications and heart rhythms, and higher temperatures might negate the protective effect.”
While the idea of cooling critically ill patients is now widely accepted, the optimal temperature to keep them at, and the method of getting them cold, is still being debated. Older methods cooled heart attack patients to 33°C, but more recent studies have shown cooling by just one degree to 36°C could be equally as effective, with fewer risks. There are also a number of different methods to get patients cold – the most simple being water-cooled blankets or adhesive pads placed on the body, with more advanced techniques involving the insertion of catheters into the body and balloons circulating ice-cold saline. Both have their pros and cons.
“The blankets are cheap, quick and less labour-intensive. But fast application does not automatically mean patients reach hypothermia more rapidly,” says Janssens. “The disadvantage of the catheters is that a trained physician has to insert them.”
Throughout treatment, drugs must also be administered to stop patients’ natural shivering response. Common complications of being kept cold for so long include severe fever, infections and damage to the skin. After the danger period is over, patients must be warmed slowly – no fasterthan 0.5°C per hour. It’s an ordeal for the body to go through, but TTM is the only post-resuscitation technique that can significantly decrease the chance of brain damage after a cardiac arrest.
Fire and ice
With cooling equipment increasingly common in medical centres, doctors are now exploring what other conditions induced hypothermia may help to treat. Dr Sam Tisherman, a surgeon and professor of critical care medicine in Baltimore, has started trials to drastically cool patients who arrive in his emergency department bleeding to death – often from multiple gunshot wounds.
“Trauma patients normally enter cardiac arrest because they have lost so much blood there just isn’t enough for the heart to work,” says Tisherman. “The problem is we just can’t sew them up fast enough – for severe blood loss their chances of survival are around 5 to 7 per cent.”
We have someone with no pulse, who’s losing so much blood CPR is not effective. We’re just trying to buy time.
Tisherman’s experimental technique involves pumping ice-cold saline directly into the body to replace lost blood, inducing a very deep state of hypothermia – as low as 15°C – not unlike the state known in science fiction as ‘suspended animation’. This is not cooling by a few degrees after a controlled cardiac resuscitation; it’s more like freezing someone and operating on them while they’re technically dead.
“The issue for us is time,” says Tisherman. “This is very different from teams who have resuscitated someone having a cardiac arrest and are trying to protect the brain from damage. We have someone with no pulse, who’s losing so much blood CPR is not effective. We’re just trying to buy time.”
Somebody with no blood reaching the brain might normally expect to die or suffer irreversible brain damage within five minutes. Tisherman says his technique has enabled people to survive after up to an hour of surgery before being slowly warmed and revived. “In the lab, we’ve even seen as much as two or three hours,” he says.
Tisherman believes it may be possible to use cooling to treat a range of other conditions, even at the scene of an emergency. “There are now teams that will try to start the cooling outside the hospital,” he says. “There’s an image being shared online of paramedics in Germany attending to a man suffering cardiac arrest in a grocery store. They piled bags of frozen French fries on him to cool him down.”
A range of studies are now investigating cooling as a way to protect against damage caused by conditions as varied as head injuries, meningitis, spinal cord injuries and liver failure. In the US, a woman arriving at hospital clinically brain-dead after committing suicide with a cocktail of sedatives and antifreeze was successfully ‘managed’ with therapeutic hypothermia for 36 hours while doctors worked on her. She awoke within 48 hours of being warmed up and made a full recovery.
The power of cold to stave off death might even mean we need to re-evaluate our definition of death. Researchers such as Dr Sam Parnia, a professor of critical care medicine, have suggested that techniques like therapeutic hypothermia are making it difficult to tell what ‘dead’ really means. In his book Erasing Death, Parnia argues that currently, the point at which medical staff stop trying to revive patients and declare them dead is entirely arbitrary.
In the near future, Tisherman hopes that the miraculous effects of cold temperatures could be replicated by a more practical medical equivalent. “We don’t use the term ‘hypothermia’, because the hope is that we could eventually find a drug that stops the brain and body needing oxygen like cold does,” he says. “That would be a lot easier.”
- This article was first published in the April 2018 of BBC Focus Magazine.
How therapeutic hypothermia works
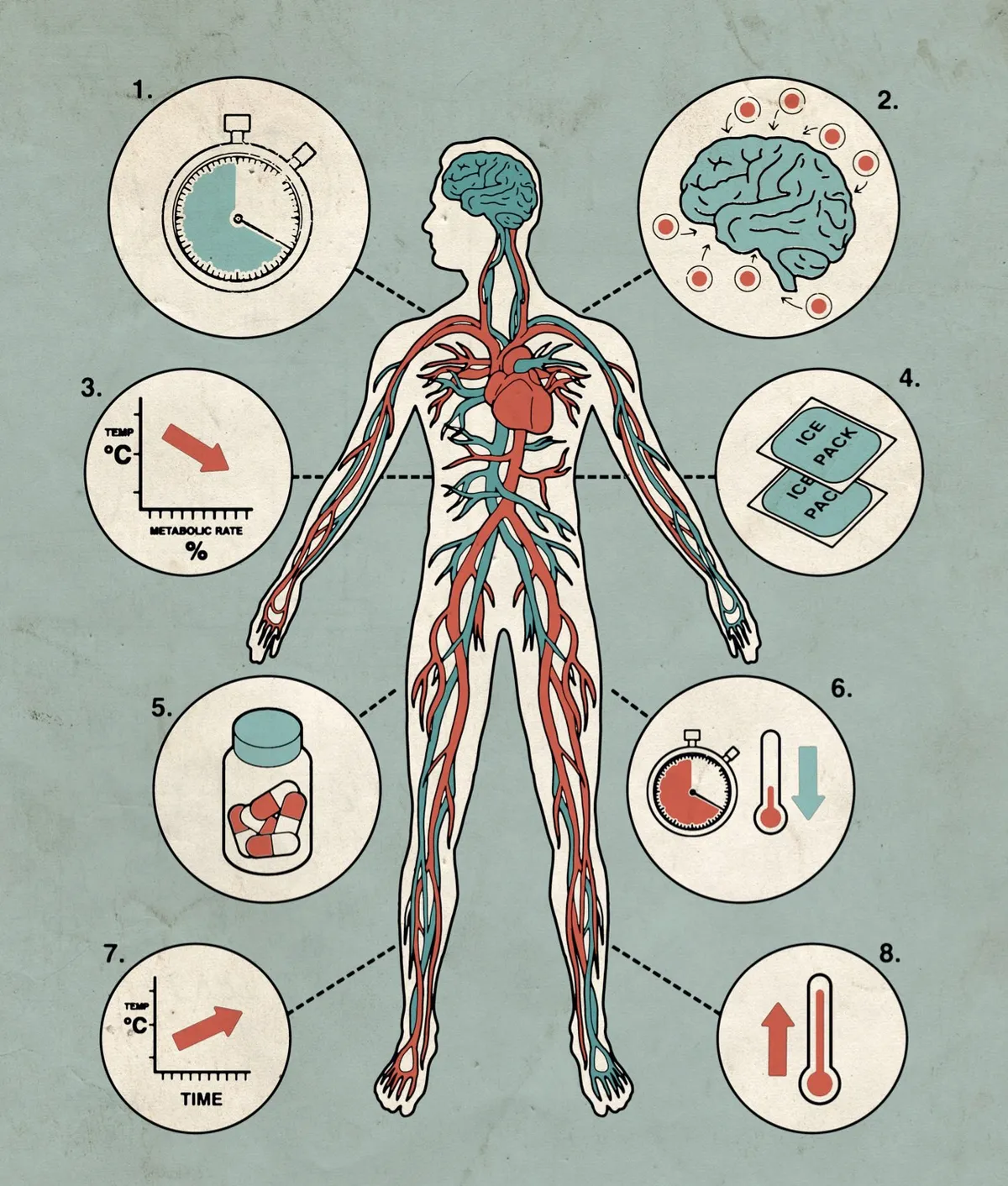
- Within the first 20 seconds of cardiac arrest, tissues start to deplete their reserves of oxygen, and toxins build up in the brain.
- If blood flow is restored, tissue damage in the brain continues. Such ‘reperfusion injury’ is thought to be caused by the production of free radicals when blood returns to oxygen-depleted cells.
- Cooling the body after cardiac arrest reduces the brain’s metabolic rate, slowing reactions that cause cell damage. For each 1°C drop in core temperature, the brain’s metabolic rate decreases by 6-7 per cent.
- Doctors use cooling blankets, pads or ice-cold instruments to reduce the body temperature to 33-36°C. Cruder temporary measures, such as applying bags of frozen food, have also been employed.
- After resuscitation, patients are kept cold and unconscious for 12-24 hours. This prevents brain damage when patients are revived. Drugs prevent shivering, which raises body temperature.
- The sooner patients can be cooled the better, although cooling patients after they have been resuscitated can still be beneficial.
- Once stable, the patient is warmed slowly – sometimes by as little as 0.1°C every hour.
- After being sufficiently warmed, the patient is revived. Patients who’ve been hypothermic for many hours will often show signs of fever, or develop respiratory infections.
Read more: